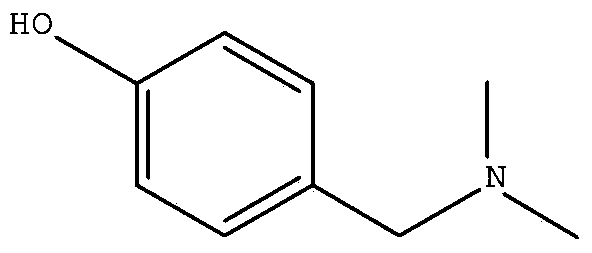
Hordenine synthesis method
A synthetic method and technology of barley base, which is applied in the field of chemical synthesis of natural product barley base, can solve the problems of low quality, excessive formaldehyde residue, and yellowish color of the product, and achieve simple operation, easy-to-obtain raw materials, and cheap and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
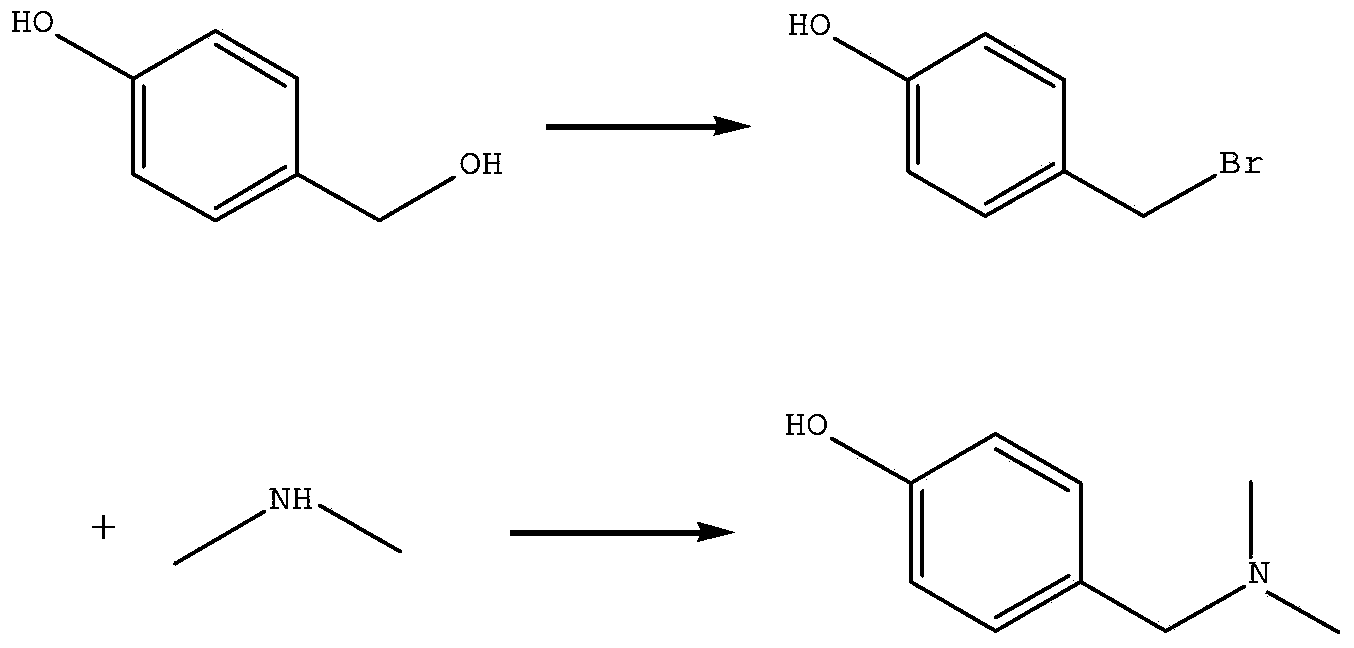
[0020] Firstly, the intermediate 4-bromoethylphenol is prepared: the raw material p-hydroxyphenethyl alcohol and 48% HBr are fed according to the feeding amount (g:ml) 1: (5-10) to prepare the intermediate 4-bromoethylphenol. The reaction temperature range is 50-100° C., and the reaction time is 10-28 hours.
[0021] Then carry out the synthesis of barley base: the intermediate 4-bromoethylphenol, the dimethylamine methanol solution with a content greater than 45% is fed according to (g:ml) 1: (3~15), or the 4-bromoethylphenol Add dimethylamine gas into the methanol solution to prepare the product barleyine. The reaction temperature range is 20-50° C., and the reaction time is 4-24 hours.
[0022]
[0023] In the present invention, the preparation of the intermediate 4-bromoethylphenol adopts cheap and easy-to-purchase p-hydroxyphenethyl alcohol as raw material for selective bromination. In the bromination method, bromination can be carried out with bromine under the cond...
Embodiment 1
[0026] 1] Preparation of 4-bromoethylphenol
[0027] Take 50g of p-hydroxyphenylethanol, dissolve it in 250ml HBr solution with a concentration of 48%, raise the temperature to 80°C, stir for 16h, cool to room temperature, filter, wash with water until neutral, and dry to obtain 72g of white solid.
[0028] 2] Preparation of hordeline
[0029] Take 20g of 4-bromoethylphenol prepared in step 1, 100ml of 45% dimethylamine methanol solution, stir and react at room temperature for about 18h, stop the reaction after TLC detects that the reaction is complete, filter, and recycle the filtrate to dryness under reduced pressure to obtain The light yellow solid was refined with ethanol water to obtain 19.2 g of pure barleyine.
Embodiment 2
[0031] 1] Preparation of 4-bromoethylphenol
[0032] Take 50g of p-hydroxyphenylethanol, dissolve it in 286ml HBr solution with a concentration of 48%, raise the temperature to 75°C, stir for 24h, cool to room temperature, filter, wash with water until neutral, and dry to obtain 73.5g of white solid.
[0033] 2] Preparation of hordeline
[0034] Take 20 g of 4-bromoethylphenol prepared in step 1 and dissolve in 100 ml of anhydrous methanol, feed dimethylamine gas to saturation at normal temperature, stop ventilation, stir for 8 h, stop the reaction after TLC detects that the reaction is complete, filter, and the filtrate reduces The solvent was recovered under pressure to dryness to obtain a light yellow solid, which was purified with ethanol and water to obtain 18.4 g of pure product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com