Method for synthesizing 3-methylquinoline-8-sulfonyl chloride
A technology of methylquinoline and a synthesis method, applied in the chemical field, can solve the problems of imperfect post-processing, cumbersome operation, low yield and the like, and achieve the effects of high practical value, good economic benefit and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
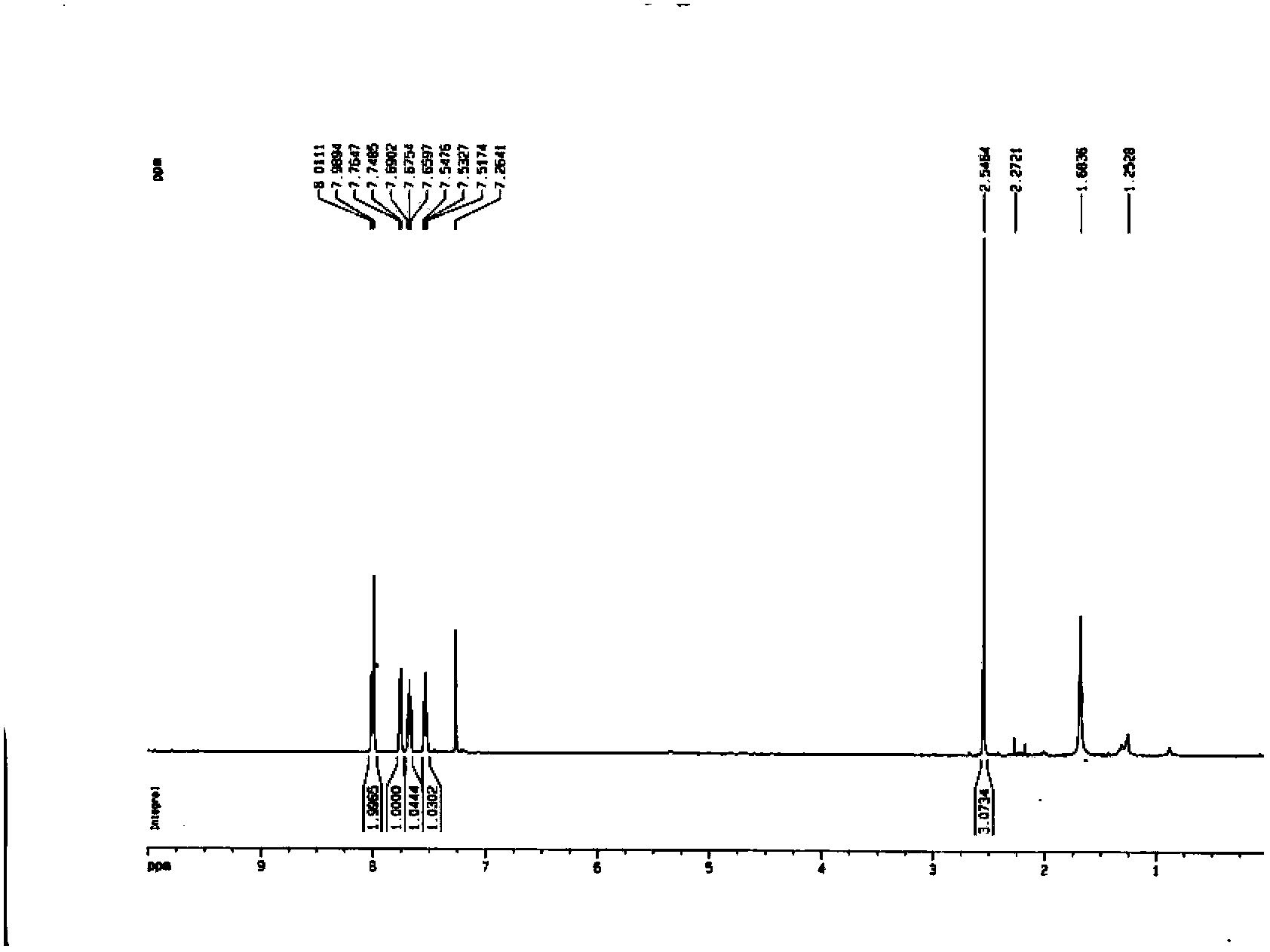
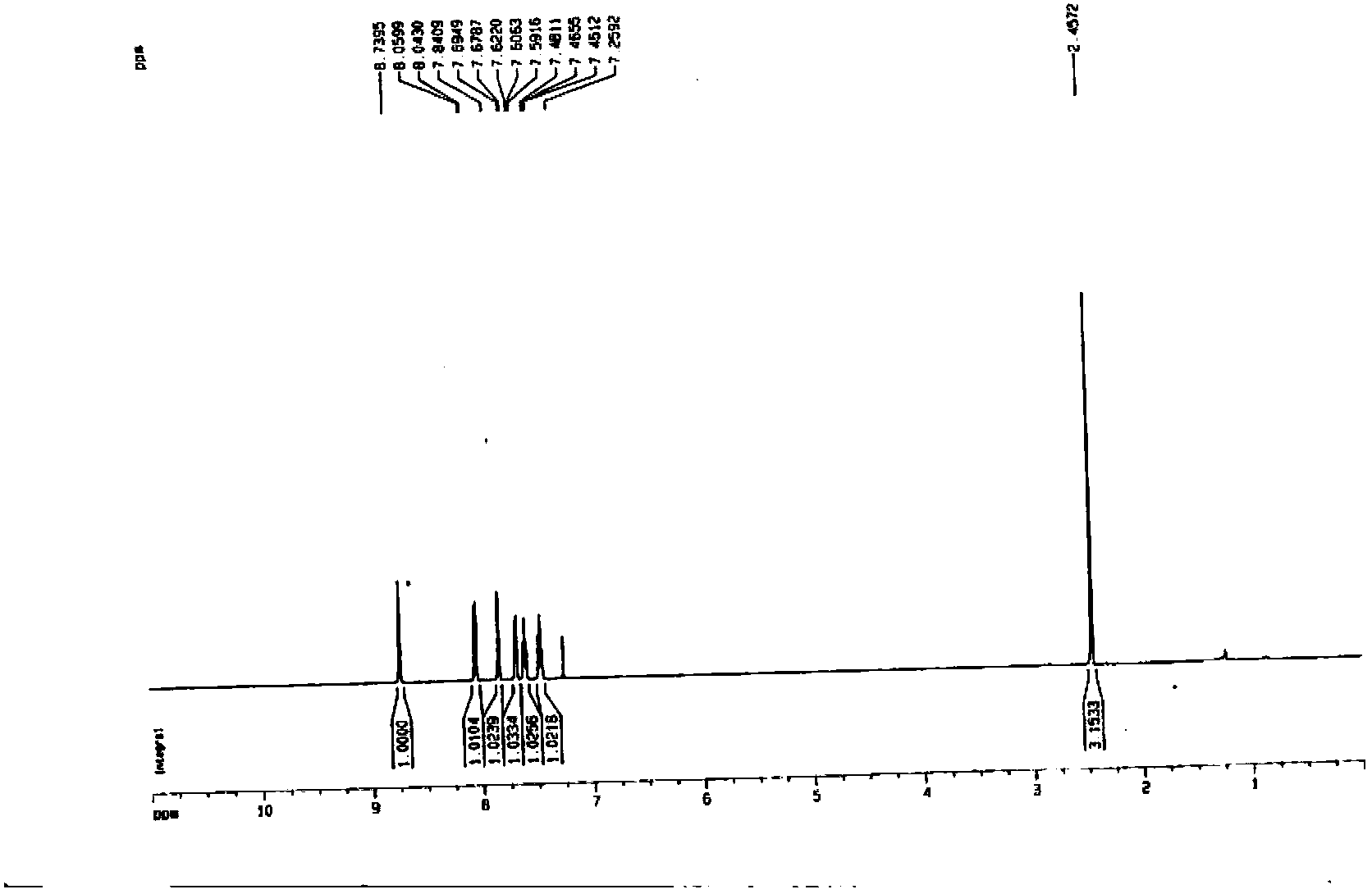
[0040] The synthetic method of 3-methylquinoline-8-sulfonyl chloride of the present invention, take aniline as raw material and obtain N-phenylpropanamide through reacting with propionic acid, then form a ring to obtain 2-chloro-3-methylquinoline, After reduction with halogenated hydrocarbons, 3-methylquinoline is obtained, and then through chlorosulfonation to obtain the product 3-methylquinoline-8-sulfonyl chloride. The inventive method specifically comprises the following steps:
[0041] The first step is to prepare N-phenylpropanamide (as shown in the following formula (2)) with aniline (as shown in the following formula (1)) as a starting material
[0042]
[0043] React aniline, propionic acid and vitamin B1, heat to 120°C, react for 10 hours, stop heating, cool to room temperature, slowly pour the reaction system into ice water with stirring, stir for 15 minutes, filter the precipitated solid, wash with water twice, Then, beating and filtering with water, the solid ...
Embodiment 1
[0060] Synthesis of N-phenylpropanamide
[0061] Connect a thermometer to a 500mL three-necked flask, reflux the condenser, add aniline (100g, 1.07mol), propionic acid (90mL, 1.2mol), vitamin B1 (10g, 34mmol) at one time, heat at 120°C, and react for 10 hours. After the reaction is over, slowly pour the reaction system into 500 mL of ice water with stirring, stir for 15 min, filter the precipitated solid, wash twice with 2x100 mL of water, make a pulp and then filter, and dry the solid at 60 ° C. The light yellow solid N-phenylpropane Amide, 90% yield.
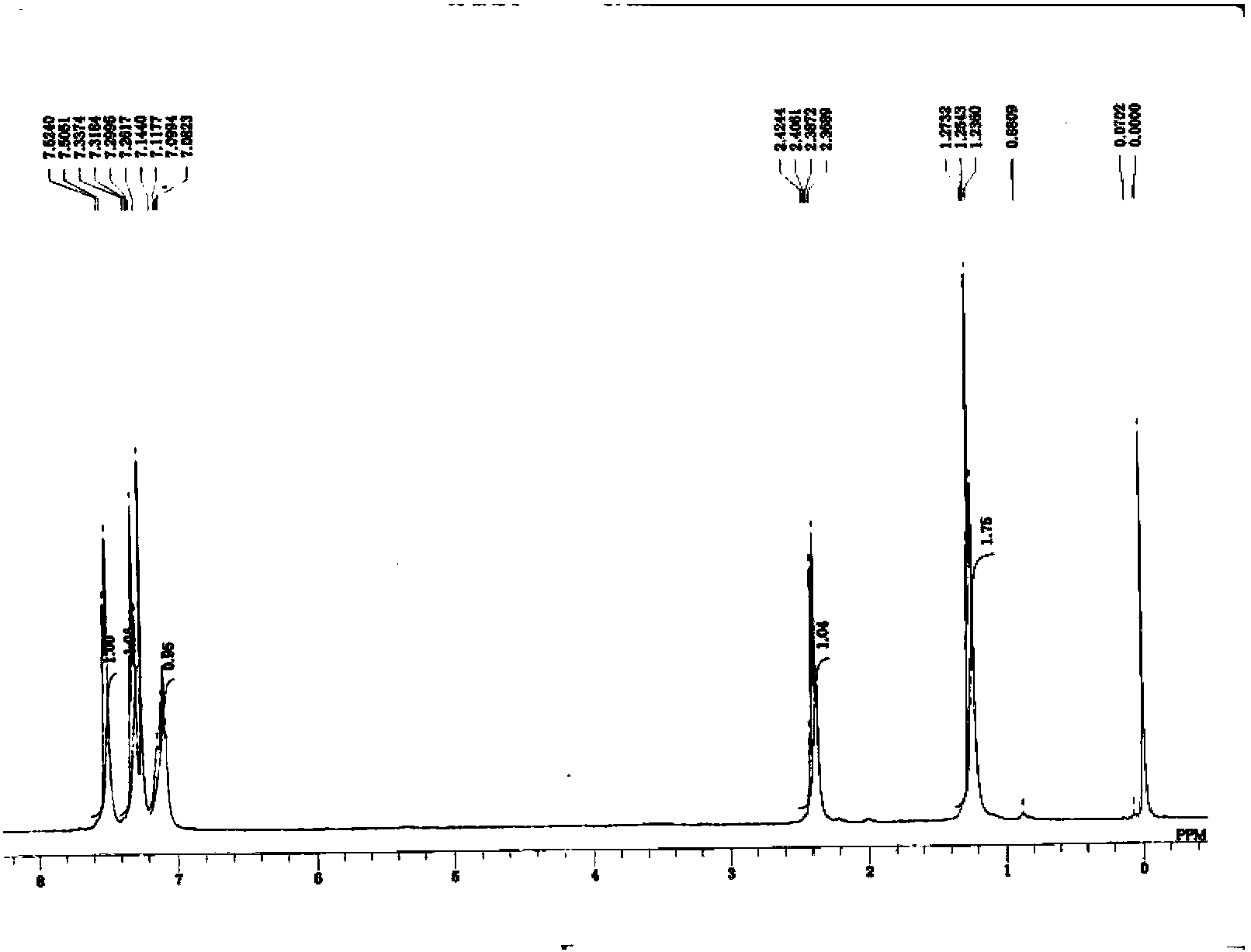
[0062] The nuclear magnetic spectrum of the N-phenylpropanamide prepared by the present embodiment is as follows figure 1 shown.
[0063] 1 HNMR (400MHz, CDCl 3 ) 7.5(d, 2H), 7.3(t, 2H), 7.1(s, 1H), 7.08~7.1(t, 1H), 2.36~2.42(m, 2H), 1.2(t, 3H).
[0064] Synthesis of 2-chloro-3-methylquinoline
[0065] Connect a thermometer, a reflux condenser, and a conduit to a 250mL three-necked flask. One end of the conduit is connec...
Embodiment 2
[0080] Synthesis of N-phenylpropanamide
[0081] Connect a thermometer to a 500mL three-necked flask, reflux the condenser, add aniline (100g, 1.07mol), propionic acid (110mL, 1.5mol), vitamin B1 (10g, 34mmol) at one time, heat at 100°C, and react for 10 hours. After the reaction, the reaction system was poured into 500mL of water, stirred for 15min, the precipitated solid was filtered, washed twice with 2x100mL of water, and the solid was dried at 60°C. The yellow solid N-phenylpropanamide yielded 85%.
[0082] Synthesis of 2-chloro-3-methylquinoline
[0083] Connect a thermometer, a reflux condenser, and a conduit to a 250mL three-necked flask. One end of the conduit is connected to a water tank, and N, N-dimethylformamide (12.20mL, 0.15mol) is added. Add oxalyl chloride (57mL, 0.6mol) dropwise, solidification will occur during the dropwise addition, continue to drop and the solidification will disappear, then add N-phenylpropanamide (15g, 0.1mol), after the addition, store...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com