Lithium nickel cobalt manganese positive electrode material and preparation method thereof
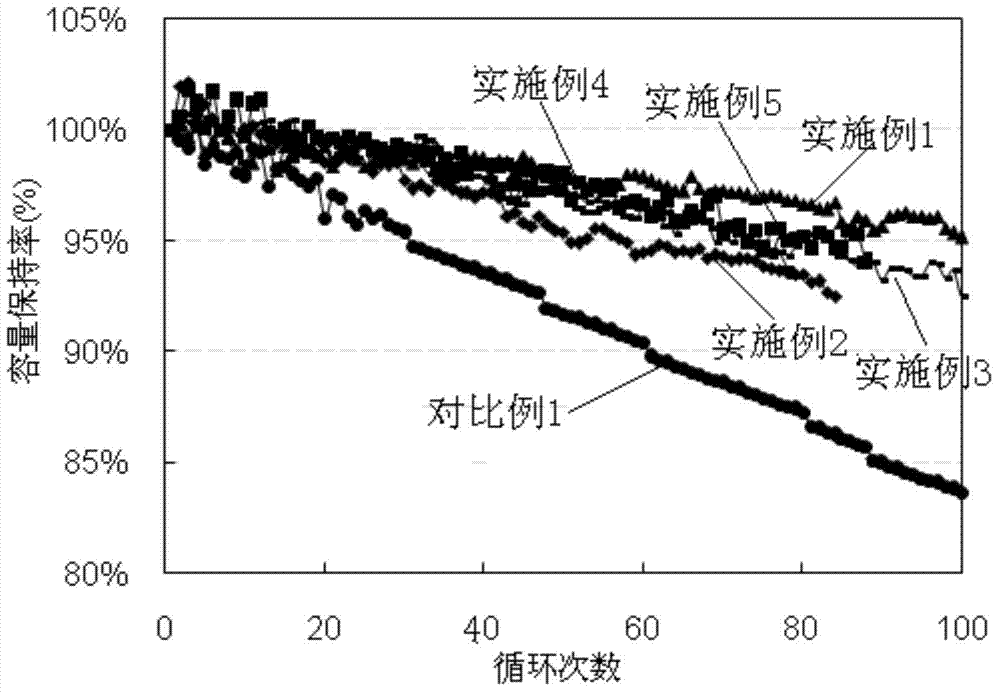
A technology of lithium-nickel-cobalt-manganese and cathode materials, which is applied in the field of lithium-ion batteries, can solve the problems of little improvement in cycle performance, reduction in capacity and rate performance, and failure to achieve comprehensive performance of the battery, achieving excellent cycle performance, high specific capacity, Ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
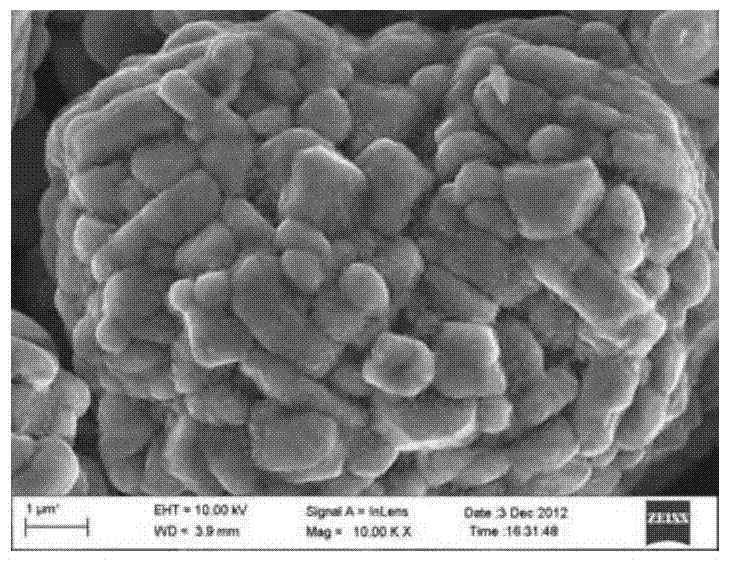
[0031] 1) According to the molecular formula Li(Ni 1 / 3 co 1 / 3 mn 1 / 3 )O 2 The proportion of transition metals to prepare NiSO 4 、CoSO 4 , MnSO 4 mixed aqueous solution, wherein the concentration of cations is 2mol / L;
[0032] 2) Add the mixed aqueous solution in step 1), 2mol / L NaOH solution, and 3mol / L ammonia solution dropwise into the reaction vessel, control the pH value of the system at 11.0, heat the water bath to 50°C, and react for 12 hours, filter, After washing and vacuum drying at 120 °C for 8 h, the precursor (Ni 1 / 3 co 1 / 3 mn 1 / 3) (OH) 2 , its D50=5μm;
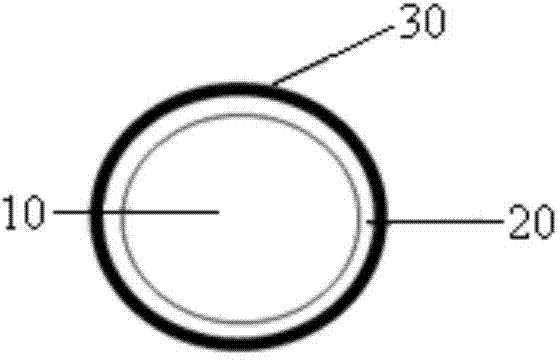
[0033] 3) the precursor (Ni 1 / 3 co 1 / 3 mn 1 / 3 )(OH) 2 1. Aluminum nitrate nonahydrate and urea nonahydrate are precursors in molar ratio: Al: urea = 100: 0.5: 8.35 The ratio of 50g precursor, 3.67g aluminum nitrate nonahydrate, and 5.35g urea nonahydrate are dissolved in 250mL. In ionized water, stir to disperse evenly and heat up to 70°C, keep stirring for 1h, filter to obtain Al(OH) 3 Coated (Ni ...
Embodiment 2
[0037] 1) According to the molecular formula Li(Ni 1 / 3 co 1 / 3 mn 1 / 3 )O 2 The proportion of transition metals to prepare NiSO 4 、CoSO 4 , MnSO 4 mixed aqueous solution, wherein the concentration of cations is 2mol / L;
[0038] 2) Add the mixed aqueous solution in step 1), 2mol / L NaOH solution, and 3mol / L ammonia solution dropwise into the reaction vessel, control the pH value of the system at 11.0, heat the water bath to 50°C, and react for 12 hours, filter, After washing and vacuum drying at 120 °C for 8 h, the precursor (Ni 1 / 3 co 1 / 3 mn 1 / 3) (OH) 2 , its D50=8μm;
[0039] 3) The precursor (Ni 1 / 3 co 1 / 3 mn 1 / 3) (OH) 2 , magnesium nitrate and dispersant urea are precursors in molar ratio: Mg: urea = 100: 0.01: 8.35 ratio, weigh 50g precursor, 3.67g magnesium nitrate and 5.35g dispersant urea and dissolve them in 250mL deionized water, stir Disperse evenly and heat up to 70°C, keep stirring for 1h, filter to obtain Mg(OH) 2 Coated precursor with D50=10μm;
[0...
Embodiment 3
[0043] 1) According to the molecular formula Li(Ni 1 / 3 co 1 / 3 mn 1 / 3 )O 2 The proportion of transition metals to prepare NiSO 4 、CoSO 4 , MnSO 4 mixed aqueous solution, wherein the concentration of cations is 2mol / L;
[0044] 2) Add the mixed aqueous solution in step 1), 2mol / L NaOH solution, and 3mol / L ammonia solution dropwise into the reaction vessel, control the pH value of the system at 11.0, heat the water bath to 50°C, and react for 12 hours, filter, After washing and vacuum drying at 120 °C for 8 h, the precursor (Ni 1 / 3 co 1 / 3 mn 1 / 3) (OH) 2 , its D50=6μm;
[0045] 3) 50g precursor (Ni 1 / 3 co 1 / 3 mn 1 / 3) (OH) 2 Disperse in a mixed solution of 50ml of absolute ethanol and 5ml of deionized water, then dropwise add 0.026M tetrabutyl titanate in isopropanol solution, stir for 1h after the dropwise addition, and filter to obtain Ti(OH) 4 Coated precursor with D50=8μm;
[0046] 4) Ti(OH) obtained in step 3) 4 Coated precursor powder with 40.76gLi 2 CO 3 T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com