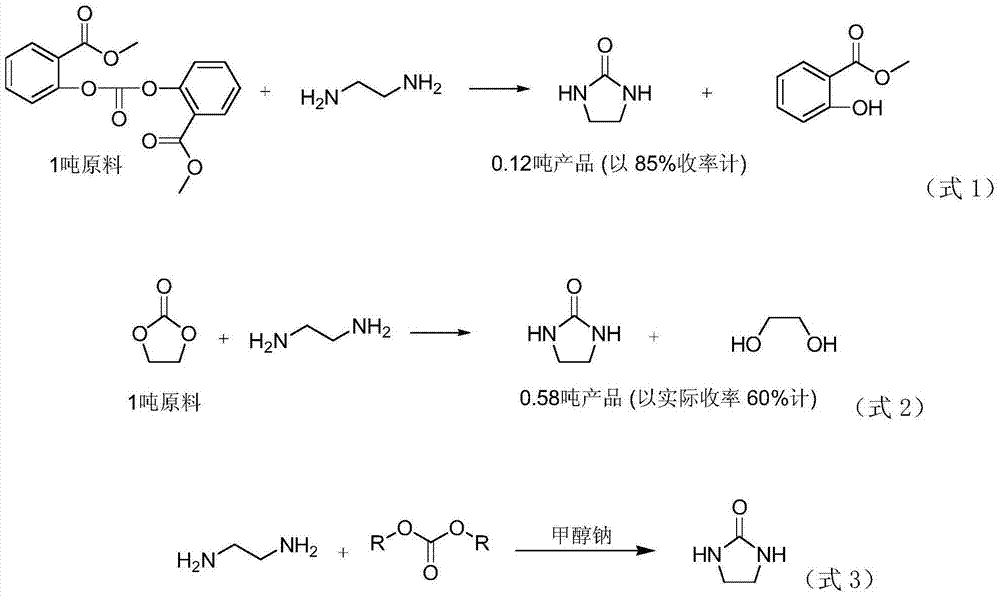
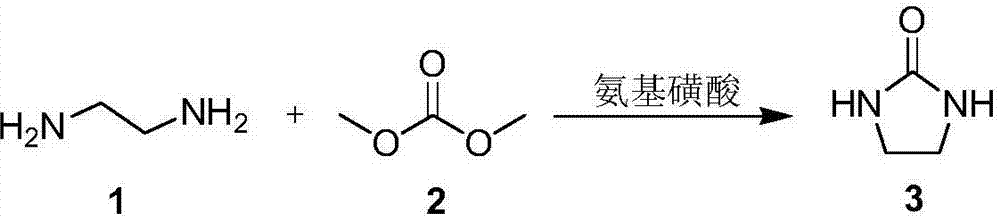
2-imidazolidone synthesis method
A technology of imidazolidinone and synthesis method, which is applied in the field of synthesis of 2-imidazolidinone, can solve the problems of expensive raw materials, relatively expensive ethylene carbonate, and high boiling point of by-products, and achieve simple and easy-to-operate reaction process and synthetic method The effect of green cleaning and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Into the heatable reactor (with stirring device), add 1.0g sulfamic acid (0.01mol), 6mL methanol, 3.0g ethylenediamine (0.05mol), 5.4g dimethyl carbonate (0.06mol), 60 After reacting at ℃ for 3h, the temperature was increased to 160℃, and the reaction was stopped after 15h. The reaction liquid is naturally cooled to room temperature, the catalyst is removed by filtration, and then the solvent is removed to obtain the crude product 2-imidazolidinone. The quantitative analysis was carried out by gas chromatograph, and the yield was 62.1%.
[0026] NMR analysis results:
[0027] 1 H NMR(400MHz, D 2 O): δ3.47 (s, 4H). 13 C NMR(100MHz, D 2 O): δ 40.69 (2C), 167.07 (1C).
Embodiment 2
[0029] Into the heatable reactor (with a stirring device), add 1.0g sulfamic acid (0.01mol), 6mL methanol, 3.0g ethylenediamine (0.05mol), 6.3g dimethyl carbonate (0.07mol), 60 After reacting at ℃ for 3h, the temperature was increased to 160℃, and the reaction was stopped after 15h. The reaction liquid is naturally cooled to room temperature, the catalyst is removed by filtration, and then the solvent is removed to obtain the crude product 2-imidazolidinone. Quantitative analysis by gas chromatograph showed that the yield was 63.4%.
Embodiment 3
[0031] Into a heatable reactor (with a stirring device), add 1.0g sulfamic acid (0.01mol), 6mL methanol, 3.0g ethylenediamine (0.05mol), 7.2g dimethyl carbonate (0.08mol), 60 After reacting at ℃ for 3h, the temperature was increased to 160℃, and the reaction was stopped after 15h. The reaction liquid is naturally cooled to room temperature, the catalyst is removed by filtration, and then the solvent is removed to obtain the crude product 2-imidazolidinone. The quantitative analysis was carried out by gas chromatograph, and the yield was 63.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com