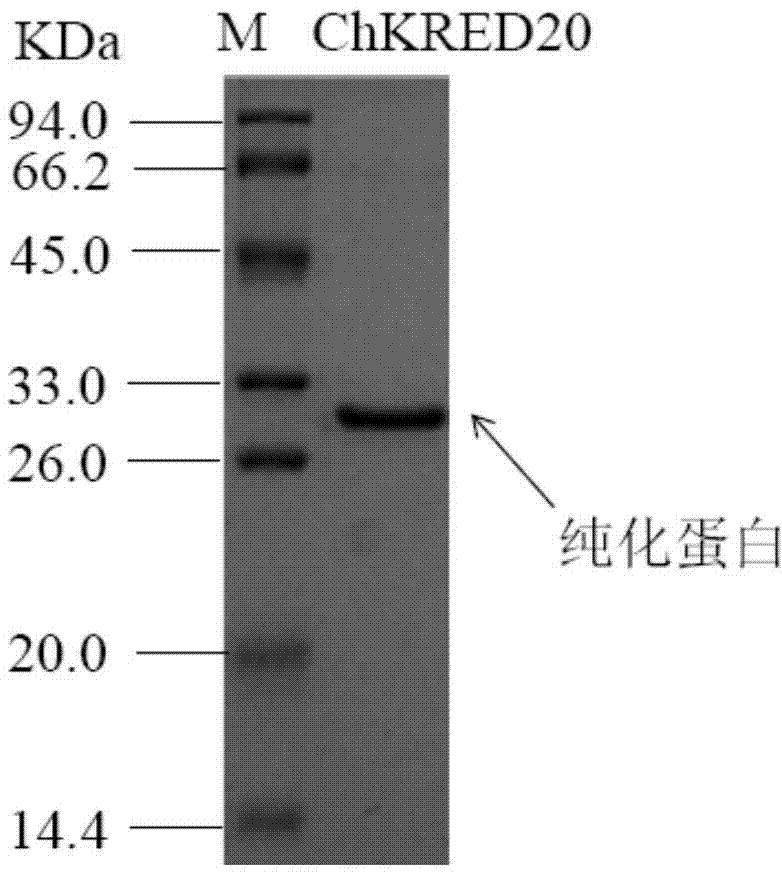
Application of Chryseobacterium sp. and carbonyl reductase thereof in production of aprepitant chiral intermediate
A kind of aureus, reductase technology, applied in the production of aprepitant chiral intermediates, the field of new carbonyl reductase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 2: the identification of screening bacterial strain
[0041] In order to confirm the taxonomic status of the strain and understand its performance in detail, the strain was initially identified. The colony of this strain is round and smooth, and with different culture time, the colony is light yellow to golden yellow.
[0042] 16S rRNA identification: Genomic DNA was used as a template, the 16S rRNA sequence was amplified with bacterial universal primers 27f and 1492r, and the PCR product was directly sent for sequencing. The obtained partial sequence (1381bp) was as follows (SEQ No.3):
Embodiment 2
[0043]AGCTGAGCGGTAGAGTTTCTTCGGAGACTTGAGAGCGGCGTACGGGTGCGGAACACGTGTGCAACCTGCCTTTATCAGGGGGATAGCCTTTCGAAAGGAAGATTAATACCCCATAATATATAGAGTGGCATCACTTTATATTGAAAACTGAGGTGGATAAAGATGGGCACGCGCAAGATTAGATAGTTGGTGAGGTAACGGCTCACCAAGTCGATGATCTTTAGGGGGCCTGAGAGGGTGATCCCCCACACTGGTACTGAGACACGGACCAGACTCCTACGGGAGGCAGCAGTGAGGAATATTGGACAATGGGTTAGCGCCTGATCCAGCCATCCCGCGTGAAGGACGACGGCCCTATGGGTTGTAAACTTCTTTTGTACAGGGATAAACCTATTTACGTGTAAATAGCTGAAGGTACTGTACGAATAAGCACCGGCTAACTCCGTGCCAGCAGCCGCGGTAATACGGAGGGTGCAAGCGTTATCCGGATTTATTGGGTTTAAAGGGTCCGTAGGCGGATCTGTAAGTCAGTGGTGAAATCTCACAGCTT AACTGTGAAACTGCCATTGATACTGCAGGTCTTGAGTAAGGTAGAAGTGGCTGGAATAAGTAGTGTAGCGGTGAAATGCATAGATATTACTTAGAACACCAATTGCGAAGGCAGGTCACTATGTCTTAACTGACGCTGATGGACGAAAGCGTGGGGAGCGAACAGGATTAGATACCCTGGTAGTCCACGCTGTAAACGATGCTAACTCGTTTTTGGGCTTTCGGGTTCAGAGACTAAGCGAAAGTGATAAGTTAGCCACCTGGGGAGTACGAACGCAAGTTTGAAACTCAAAGGAATTGACGGGGGCCCGCACAAGCGGTGGATTATGTGGTTTAATTCGATGATACGCGAGGAACCTTACCAAGGCTTAAATGGGAATTGATCGGTTTAGAAATAGACCTTCCTTCGGGCAATTTTCAAGGTGCTGCATGGTTGTCG...
Embodiment 3
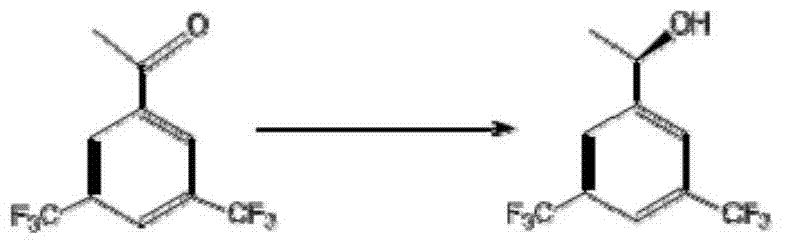
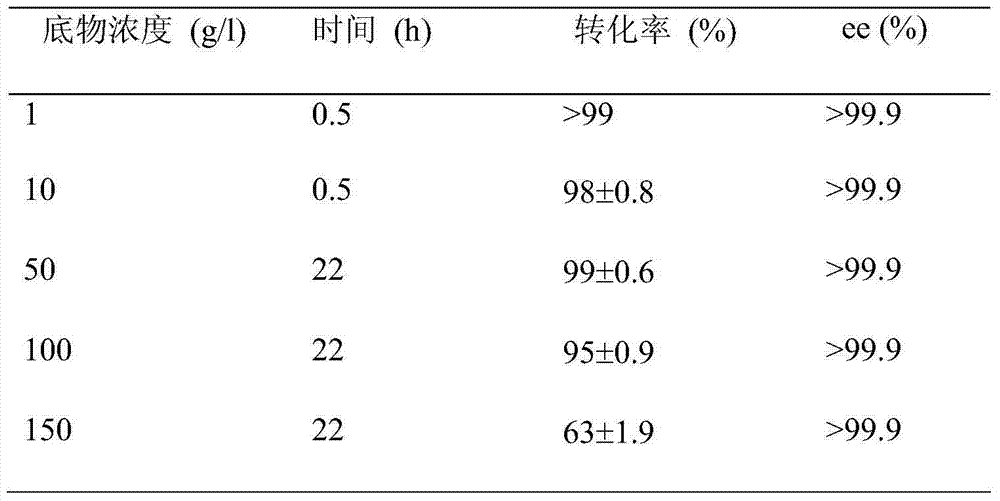
[0048] (1) Take 0.5g of bacteria and resuspend in 10ml buffer, add substrate 3,5-bistrifluoromethyl acetophenone 10mg, and add 0.5ml of isopropanol (5%) and glucose 0.2g (2% ), 30°C, 230rpm conversion for 5h. Extract with 10ml ethyl acetate, take the organic phase gas chromatography (SGE, Australia, AC-5, 30m×0.22) to determine the conversion rate is 95.6%, and determine the gas phase chiral column (CHIRASIL-DEX CB, Varian 25m×0.25) The ee value of the product is greater than 99%.
[0049]
[0050] The product data is as follows:
[0051] colorless liquid, [α] D 25 =+21.6(c=1, acetonitrile);
[0052] ee>99% (CHIRASIL-DEX CB, column temperature: 120°C, injector: 260°C, detector: 280°C, t=8.403min);
[0053] 1H NMR (600MHz, CDCl 3 ):δ7.84(s,2H,Ar-H),7.78(s,1H,Ar-H),5.04(q,J=6.54Hz,1H,-CH),1.99(br,1H,OH), 1.55(d,J=6.54Hz,3H,-CH 3 )
[0054] (2) Take 1.0 g of bacteria and resuspend in 10 ml of buffer, add 100 mg of substrate, add the same amount of glucose and isopropa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com