Fusion protein CR2-Linker-GDH and application thereof
A fusion protein and sequence technology, which is applied in the direction of fusion polypeptide, recombinant DNA technology, and the use of vectors to introduce foreign genetic material, etc., can solve the problems of high cost, troublesome operation, and low efficiency of coenzyme regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the acquisition of fusion gene
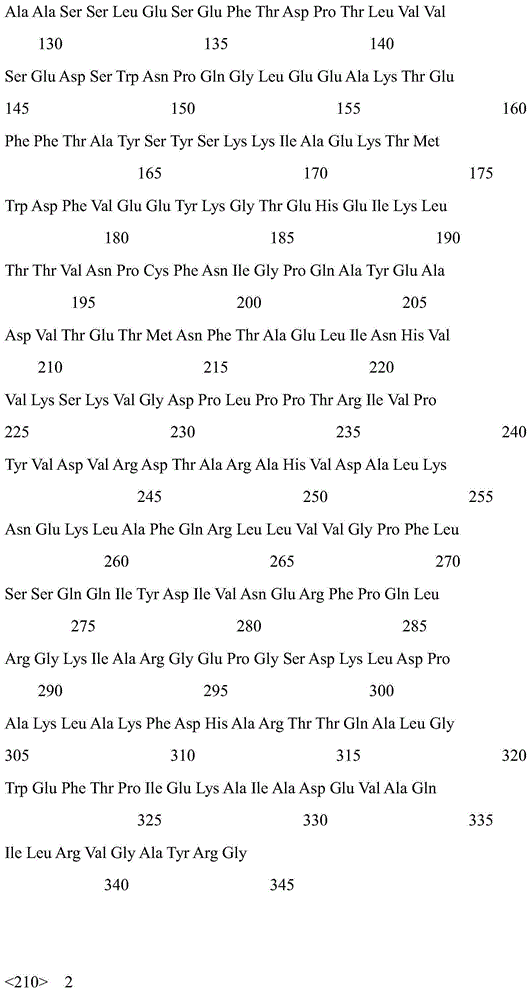
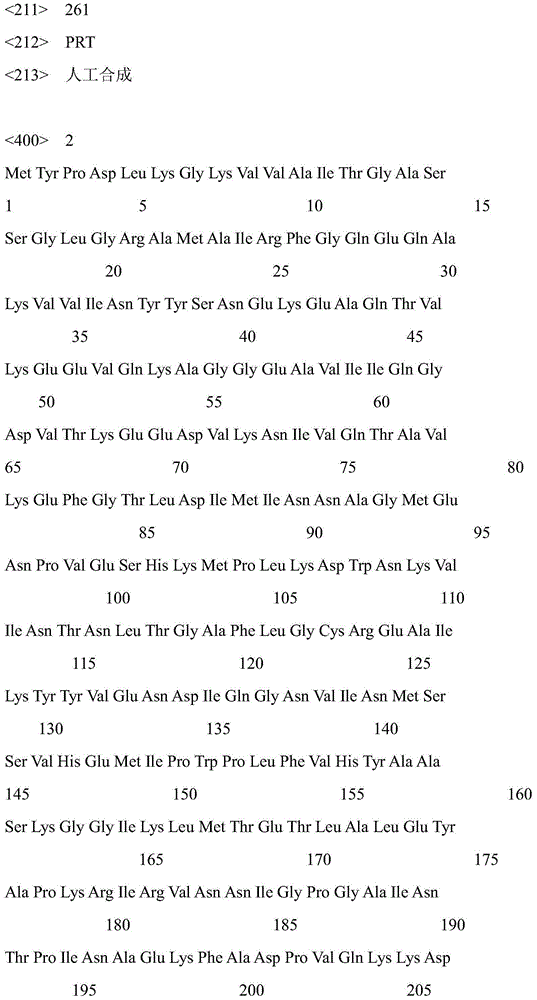
[0027] The cores of carbonyl reductase CR2 and glucose dehydrogenase GDH were obtained from E.coliBL21 / pET-CR2 and E.coliBL21 / pET-GDH by extracting plasmids, PCR amplification, or direct chemical synthesis. Nucleotide sequence (respectively shown in SEQ ID NO.5, SEQ ID NO.6), its amino acid sequence is shown in SEQ ID NO.1, SEQ ID NO.2 respectively.
[0028] A flexible oligopeptide linker (GGGGS) was added between the two genes using overlap extension PCR 3 , get the fusion expression gene cr2-linker-gdh. The nucleotide sequence of the fusion gene is shown in SEQ ID NO.4, and the encoded amino acid sequence is shown in SEQ ID NO.3.
[0029] The method for obtaining the fusion gene is specifically:
[0030] (1) Using the plasmid pET-cr2 as a template, using upstream primer 1 (sequence shown in SEQ ID NO.7) and downstream primer 2 (sequence shown in SEQ ID NO.8), amplify the cr2 gene fragment by PCR reaction ;
[0031] (...
Embodiment 2
[0033] Embodiment 2: Construction of the genetically engineered bacteria expressing fusion protein CR2-Linker-GDH
[0034] (1) After the fusion gene obtained in Example 1 is purified, it is connected to the pMD-19T carrier, transformed into Escherichia coli E.coliJM109 competent cells, and the correct recombinant plasmid pMD-C-L-G is screened, and verified by sequencing;
[0035] (2) Digest the recombinant plasmids pMD-C-L-G and pET-28a with restriction endonucleases Nde Ⅰ and Not Ⅰ respectively, recover and purify the digested products, and linearize the pET-28a vector and c-l-g gene fragments in T4 Ligated under the action of ligase to obtain a recombinant plasmid with two target genes cr2 and gdh, screened the correct recombinant plasmid and named it pET-C-L-G.
[0036] (3) Transform the competent E.coliBL21(DE3) with the recombinant plasmid pET-C-L-G obtained in the previous step to obtain the final positive strain E.coli BL21 / pET-C-L-G.
Embodiment 3
[0037] Embodiment 3: Induced expression of fusion protein
[0038]Pick a single colony of the recombinant strain E.coli BL21 / pET-C-L-G and inoculate it in 5 mL of LB liquid medium containing 50 μg / mL kanamycin, culture it overnight at 37°C with shaking at 200 rpm, and transfer 1 mL of the culture liquid to 50 ml In the LB liquid medium containing 50μg / mL kanamycin, culture at 37°C and 200rpm with shaking until the OD is 0.6-0.8, then add the inducer isopropyl-D-thiogalactoside IPTG 0~ 1mmol / L, induced culture at 17°C for 10h; centrifuge at 10,000rpm for 10min, collect the bacteria, wash twice with saline, and collect the whole cells of the recombinant bacteria. The expression of fusion protein can reach 0.143g / g (protein / cell)
[0039] Resuspend the collected whole cells of the recombinant bacteria in the injection buffer used for purification, and ultrasonically disrupt: working time 2s, intermittent time 3s, 20min in total; centrifuge the disrupted solution at 12000rpm for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com