Method for preparing dicalcium phosphate and tricalcium phosphate
A technology of tricalcium phosphate and dicalcium phosphate, which is applied in the field of calcium phosphate salts, can solve the problems of not being able to directly produce dicalcium phosphate and tricalcium phosphate, and achieve good economic and environmental benefits, simple process, and less waste discharge.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
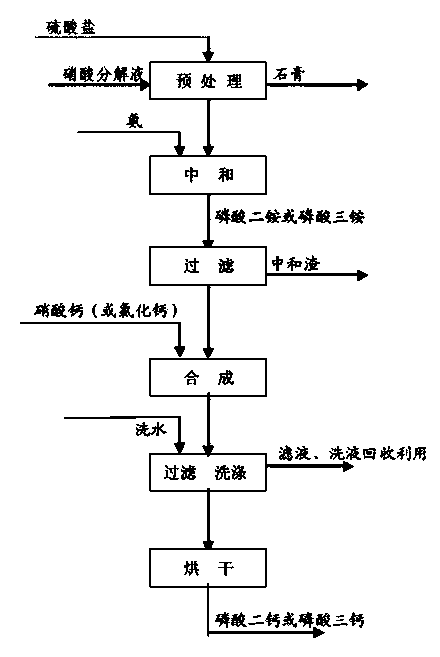
Image
Examples
Embodiment 1
[0028] Embodiment 1: Taking wet-process phosphoric acid as raw material
[0029] Table 1. Chemical composition of wet-process phosphoric acid
[0030] project P 2 o 5 CaO MgO Fe 2 o 3 Al 2 o 3 F SO 3 Mass fraction % 28.30 0.16 1.25 1.10 0.98 1.64 1.63
[0031] Add 800g of wet-process phosphoric acid into the reaction tank, add concentrated ammonia water with a mass fraction of 25% under stirring to neutralize to a pH of about 8.0, the reaction temperature is 50°C to 80°C, and the residence time is 1.5 hours, after which the neutralized slurry is filtered; the obtained Add 1000g of calcium chloride solution with a mass fraction of 20% to the filtrate under stirring, the reaction temperature is 30°C-60°C, and the residence time is 1.0 hour. Finally, the synthetic slurry is filtered, washed with water, and dried to obtain 462g of dicalcium phosphate product. The chemical composition of the product is shown in Table 2.
[0032] Ta...
Embodiment 2
[0034] Embodiment 2: the solution that decomposes phosphate rock with nitric acid is used as raw material to prepare dicalcium phosphate
[0035] Table 3 Chemical composition of nitric acid decomposition solution
[0036] project P 2 o 5 CaO MgO F % 22.4 4.30 0.78 1.82
[0037] Table 4 Chemical composition of calcium nitrate
[0038] project P 2 o 5 CaO MgO F % 0.14 19.2 0.25 0.01
[0039] Add 1000g of nitric acid solution to decompose phosphate rock and 220g of filtered gypsum lotion into the reaction tank, add 80g of ammonium sulfate under stirring, react at 30°C to 90°C for 1 hour, then filter the material and wash with water; the obtained filtrate Use 25% ammonia water to neutralize to a pH of about 8.0, the reaction temperature is 50°C to 80°C, and the residence time is 1.5 hours; then filter the neutralized slurry; mix the obtained filtrate with the dicalcium phosphate lotion and add 750g of calcium nitrat...
Embodiment 3
[0042] Embodiment 3: use the solution of nitric acid to decompose phosphate rock as raw material to prepare tricalcium phosphate
[0043] The chemical composition of the solution for decomposing phosphate rock with nitric acid is the same as in Example 2.
[0044] Add 1000g of nitric acid decomposition solution and 230g of lotion into the reaction tank, add 80g of ammonium sulfate under stirring, react at 30°C to 90°C for 1 hour, then filter the material and wash with water; neutralize the obtained filtrate with 25% ammonia water to The pH is about 14.0, the reaction temperature is 30°C-50°C, the reaction process needs to be cooled down, and the residence time is 1.5-3 hours; the neutralized slurry is filtered, the obtained filtrate is mixed with the tricalcium phosphate washing liquid, and 1100g of calcium nitrate is added under stirring. The reaction temperature is 30°C-80°C, and the residence time is 1.0-2 hours; finally, the synthetic slurry is filtered, and the filter cak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com