Synthesis method and application of chitosan oligosaccharide/indometacin graft
A technology of indomethacin grafts and synthesis methods, which can be applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of poor selectivity and insoluble in water, etc. It achieves the effects of strong inhibition rate, no organic solvent residue, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
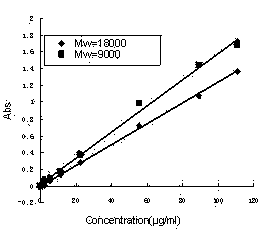
[0052] (1) Weigh carbodiimide EDC (0.5953g, 3.105mmol) and indomethacin 0.2222g (0.6210mmol) into 8ml of acetone to form A liquid, and stir the A liquid at a constant temperature of 25°C The solution dissolves. Then weigh 0.2g (0.02222mmol) of chitosan oligosaccharide (Mw=9000) and put it in 10ml of distilled water to form B solution, which is also stirred and dissolved at a constant temperature of 25°C. At 25°C, slowly drop liquid A into liquid B while liquid B is stirring. After the dropwise addition, the solution was placed on a constant temperature heating magnetic stirrer, and reacted at a reaction temperature of 25° C. for 53 hours. After the reaction, the reaction solution was transferred to a dialysis bag (membrane molecular weight cut-off MWCO=7000), and deionized water was used as the dialysate for dialysis for 10 hours. The water was changed every half hour for the first three hours, and the water was changed every hour thereafter. After the dialysis is finished, ...
Embodiment 2~7
[0055] Different molecular weight chitosan oligosaccharides and different graft ratios of indomethacin as described in table 1, according to the synthetic method of the chitosan oligosaccharide / indomethacin graft of embodiment 1, synthetically obtains chitosan oligosaccharide / indomethacin Purified products of octyl grafts 2-7.
[0056] Table 1: Chitooligosaccharides with different molecular weights and different grafting ratios of indomethacin
[0057]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com