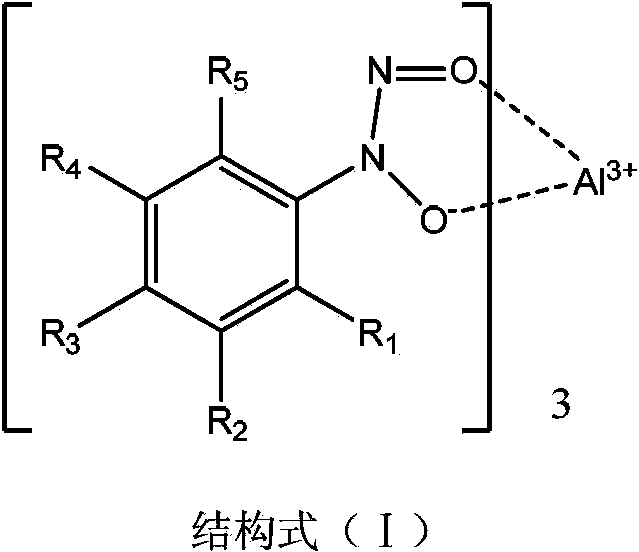
Application of aluminum salt compound as polymerization inhibitor
A technology of compound and polymerization inhibitor, which is applied in the field of tris[N-nitroso-N-(substituted phenyl)hydroxylamine]aluminum salt compound to achieve the effect of overcoming the problem of benzene molecule release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
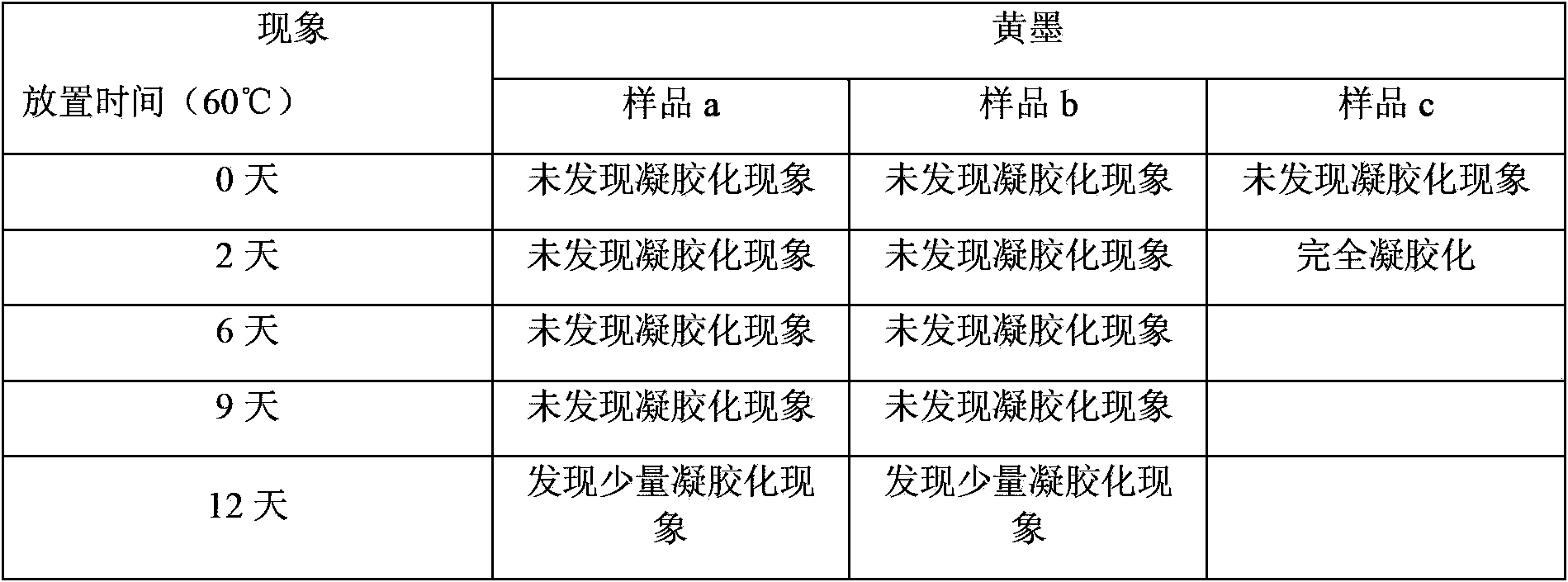
Examples
Embodiment 1
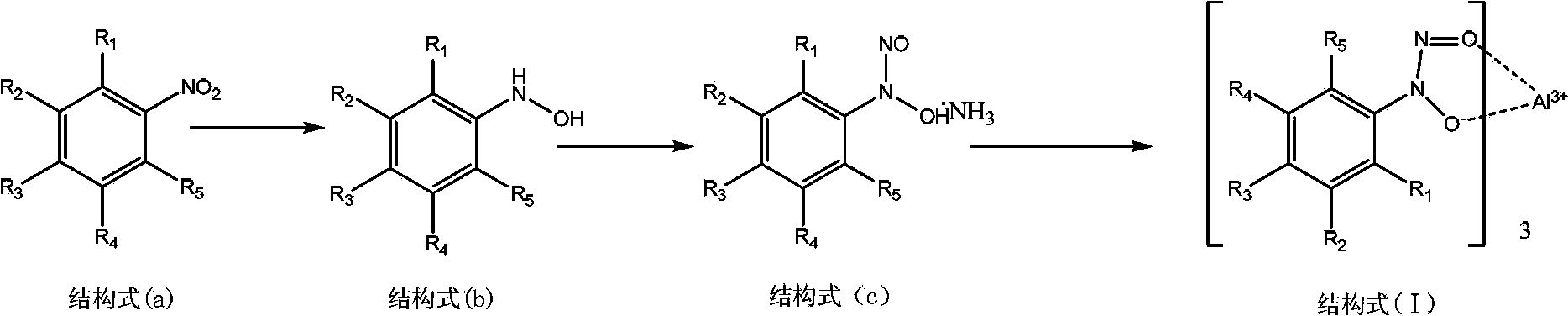
[0015] Example 1 Preparation of N-nitroso-N-(4-methylphenyl) hydroxylamine ammonium salt
[0016] Add 20g (0.146mol) of 4-nitrotoluene, 9.15g (0.171mol) of ammonium chloride, and 150ml of water into a 250ml three-necked flask under nitrogen protection, stir to dissolve, add 36g (0.551mol) of Zn powder, and control The reaction temperature is 60-70°C. The reaction was stirred after the addition was complete. After the reaction is complete, filter, and use sodium chloride to make the filtrate a saturated saline solution, add diethyl ether to extract, after the diethyl ether solution is dried, cool down to 0°C, pass through dry ammonia gas, and add dropwise 17g (0.165 mol) butyl nitrite, control the temperature below 10°C, continue to pass ammonia gas to stir the reaction after the dropwise addition, filter and wash to obtain 17.8g of white flaky crystals, the yield is 72.3%, and the melting point is 148.2-150.3°C.
Embodiment 2
[0017]Example 2 Preparation of Tris[N-nitroso-N-(4-methylphenyl)hydroxylamine]aluminum salt
[0018] In a dry and clean 50ml three-necked flask, add 8.0g of N-nitroso-N-(4-methylphenyl)hydroxylamine ammonium salt, 20g of deionized water, 20g of dichloromethane, and add a pH value of 5.0-6.0 8g of buffer solution; stirring at room temperature and adding dropwise 9g of aluminum nitrate aqueous solution (3g of aluminum nitrate nonahydrate dissolved in 6g of deionized water), stirring and reacting after the addition is completed, after the reaction is completed, let stand to separate liquid, wash with deionized water, and separate liquid; organic Dichloromethane was recovered by phase distillation, and ethanol was added to stir and crystallize to obtain a white solid, which was filtered and dried to obtain 6.96 g, with a yield of 92%. Content 99.79%, melting point: 142.4-144.9 ℃. 1 H-NMR data δ2.4037ppm(s,3H,CH 3 ); 7.2489, 7.2764ppm (d, 2H, Ar); 7.8815, 7.9088ppm (d, 2H, Ar). ...
Embodiment 3
[0019] Embodiment 3 Preparation of N-nitroso-N-(2-methylphenyl) hydroxylamine ammonium salt
[0020] Add 20g (0.146mol) of 2-nitrotoluene, 9.15g (0.171mol) of ammonium chloride, and 150ml of water into a 250ml three-necked flask under nitrogen protection, stir to dissolve, add 36g (0.551mol) of Zn powder in batches, and add Control the reaction temperature at 60-70°C. Stir the reaction after the addition is complete, and the reaction ends. Filtrate, saturate the filtrate with sodium chloride, add diethyl ether for extraction, dissolve anhydrous sodium sulfate in diethyl ether and dry, then cool down to 0°C, pass through dry ammonia gas, and add 17g (0.165mol) of newly prepared butyl nitrite dropwise after about 15 minutes Esters, control the temperature below 10°C, and continue to pass ammonia gas to stir the reaction for half an hour after the dropwise addition is completed. After filtration and washing, 9.2 g of white flaky crystals were obtained, melting point: 98.8-109.8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com