Real-time fluorescent nucleic acid thermostatic amplification detection kit of influenza virus A (H1N1) (2009)
A technology of influenza virus and H1N1, applied in the direction of fluorescence/phosphorescence, measurement/inspection of microorganisms, biochemical equipment and methods, etc., can solve problems such as complicated operation, hindering large-scale promotion and use, and contamination of amplicons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Embodiment 1, be used for the design of the special primer and probe of real-time fluorescent nucleic acid constant temperature amplification detection type A H1N1 influenza virus (2009)
[0101] The present invention selects the H1N1 (2009) virus HA gene with no secondary structure and a highly conserved segment as the amplified target sequence region (its nucleotide sequence is shown in sequence 1 in the sequence table), and according to the principle of primer probe design, use DNAATAR, DNAman software and artificially designed special primers and probe sequences for real-time fluorescent nucleic acid constant temperature amplification to detect influenza A (H1N1) virus (2009) obtained the following specific sequences:
[0102] (1) a capture probe (TCO, Target Capture Oligo) that can specifically combine with the target nucleic acid (H1N1 (2009) RNA) sequence of the influenza A H1N1 influenza virus (2009) shown in sequence 1 in the sequence listing, said The nucleoti...
Embodiment 2
[0106] Example 2, preparation of influenza A H1N1 virus (2009) real-time fluorescent nucleic acid constant temperature amplification detection kit
[0107] Using the special primers and probes provided in Example 1, a real-time fluorescent nucleic acid constant temperature amplification detection kit for influenza A (H1N1) virus (2009) of the present invention was obtained. The kit contains capture probe (TCO, Target Capture Oligo), T7 primer, nT7 primer, H1N1 (2009) detection probe, M-MLV reverse transcriptase and T7 RNA polymerase; when there is an internal standard in the kit, Internal standard detection probes are also included.
[0108] The capture probe exists in the nucleic acid extraction solution, the T7 primer, nT7 primer, H1N1 (2009) detection probe, and internal standard detection probe exist in the H1N1 (2009) detection solution, and the M-MLV reverse Recording enzyme and T7 RNA polymerase exist in the SAT enzyme solution. Specifically, the kit is divided into A ...
Embodiment 3
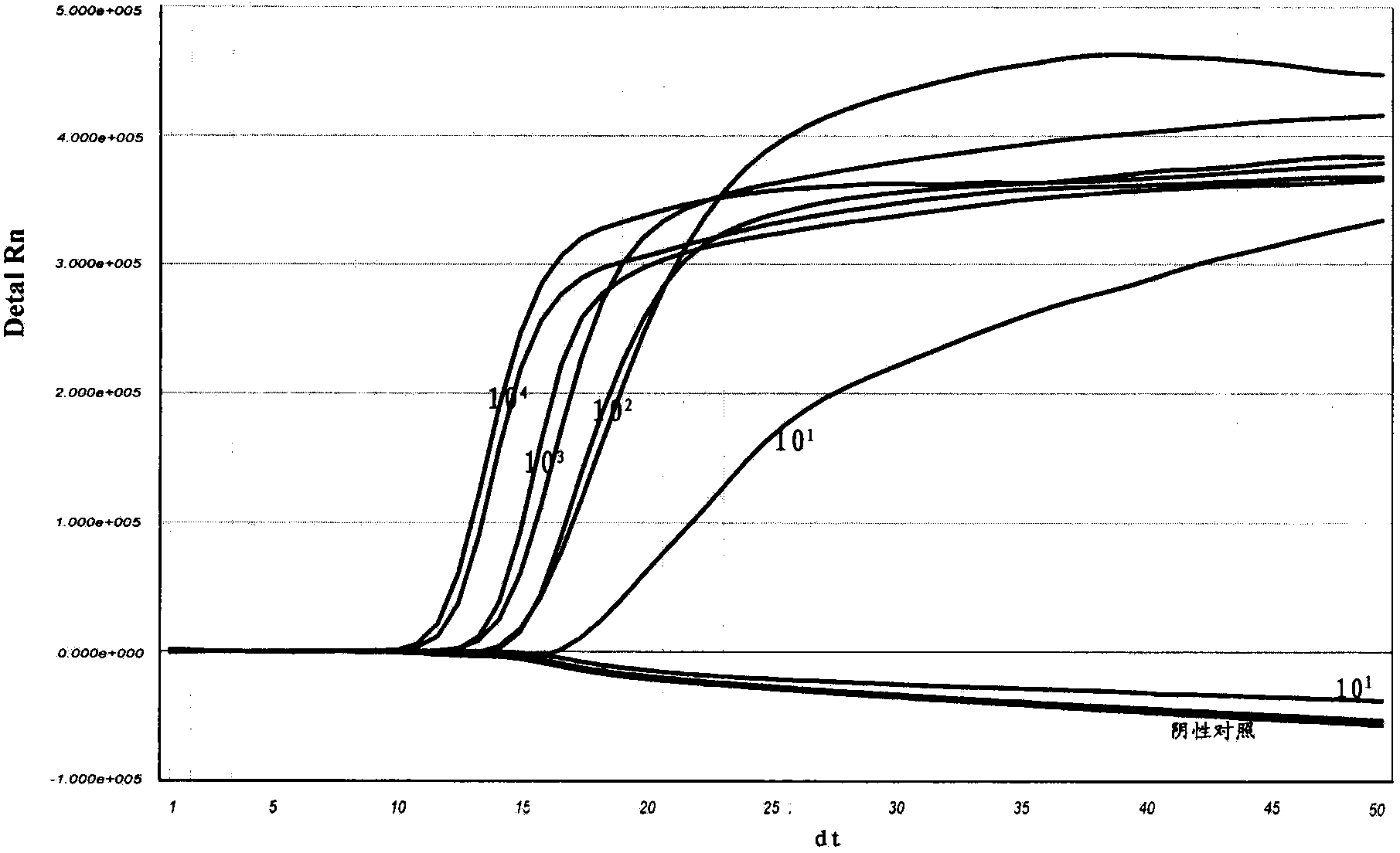
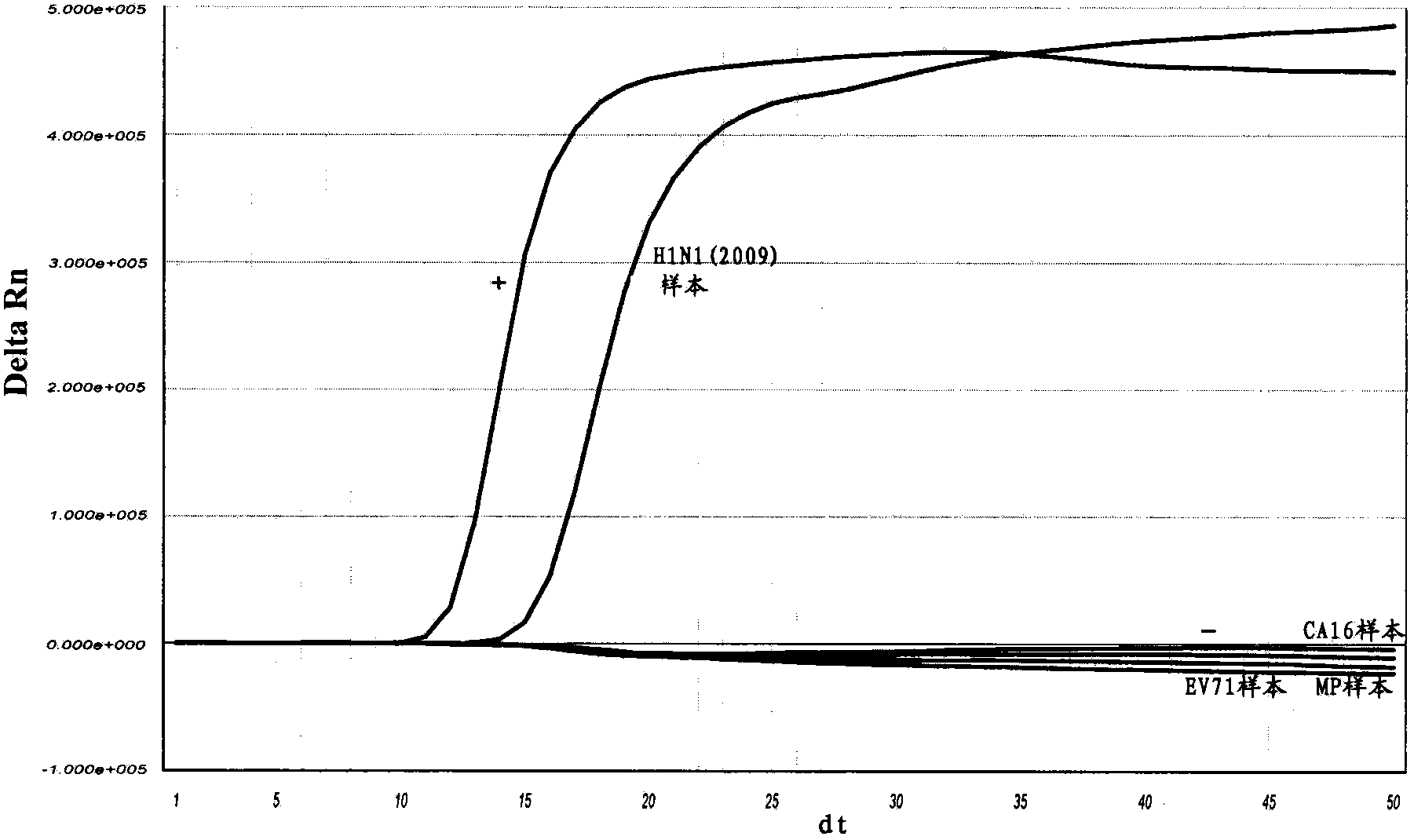
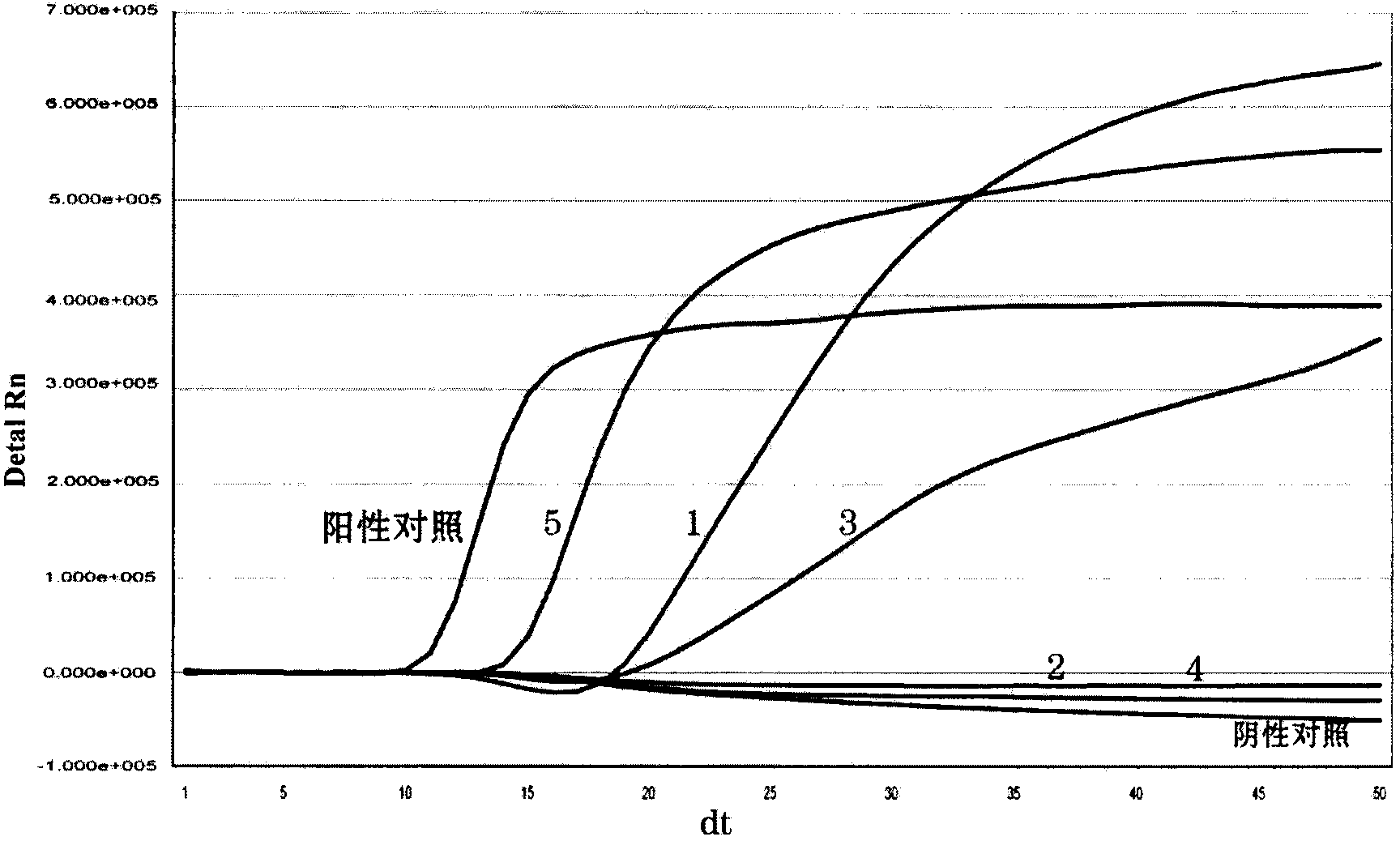
[0141] Embodiment 3, the detection sensitivity of real-time fluorescent nucleic acid constant temperature amplification of influenza A (H1N1) virus (2009)
[0142] With the kit of the present invention (see Example 2 for the composition, there is no H1N1 (2009) internal standard in the kit, and there is no internal standard detection probe in the detection solution) the measured concentration is 1 × 10 4 copies / reaction, 1×10 3 copies / reaction, 1×10 2 Copies / reaction, 10copies / reaction of Influenza A H1N1 virus (2009) positive control RNA to determine the lower limit of sensitivity. Specific steps are as follows:
[0143] (1) dilution
[0144] Will 1×10 4 Influenza A H1N1 virus (2009) positive reference RNA of copies / reaction, 10-fold serial dilution to 1×10 3 copies / reaction, 1×10 2 copies / reaction, 10copies / reaction.
[0145] (2) Nucleic acid extraction
[0146] 2.1 Add 200 μl lysate (containing HEPES 35mM, (NH 4 ) 2 SO 4 20mM), 200 μl influenza virus positive re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com