Carbon-coated lithium-rich positive electrode material as well as preparation method thereof
A technology of lithium-rich cathode material and lithium-rich material, applied in battery electrodes, electrical components, circuits, etc., can solve the problem that transition metals cannot be oxidized, and achieve the effects of low raw material cost, convenient large-scale industrial production, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
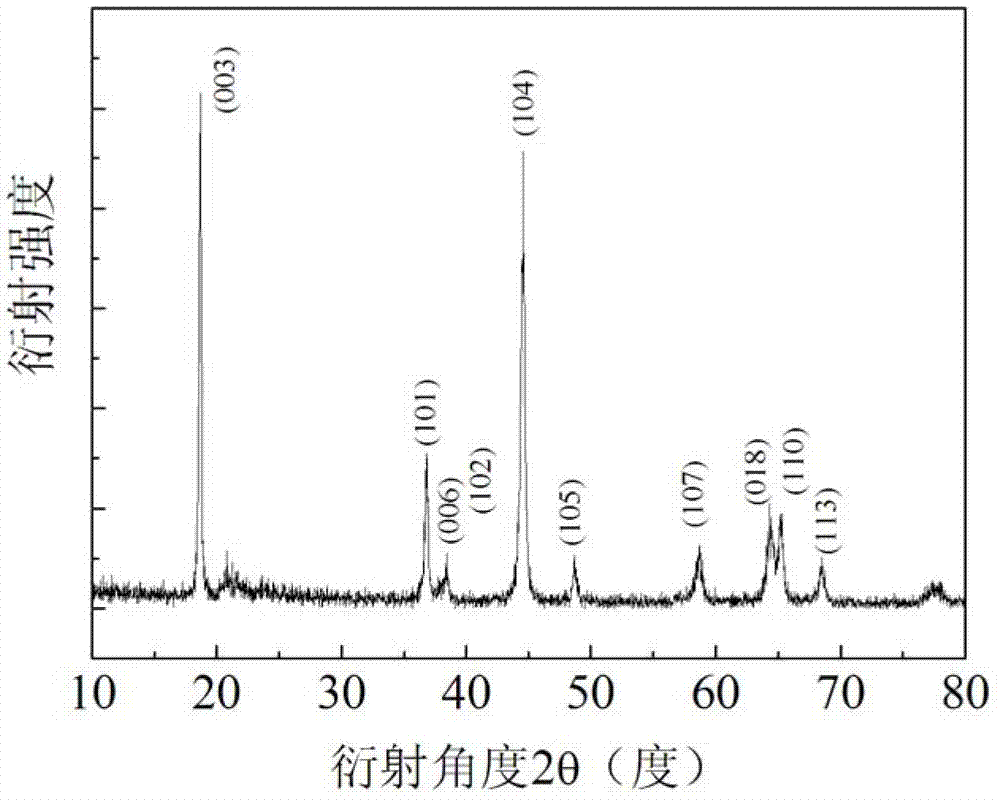
[0023] Weigh 3.74g Ni(CH 3 COO) 2 4H 2 O and 8.58g Mn(CH 3 COO) 2 4H 2 O was dissolved in 100 mL of ethanol deionized water (the volume ratio of ethanol to deionized water was 3:1), and magnetically stirred at room temperature for 2 h to fully dissolve it. Prepare ammonia water with a concentration of 0.5 mol / L, add the ammonia water dropwise to the metal salt solution, and adjust its pH value to 11. Take 7.02mL of hydrogen peroxide and add it dropwise to the above solution. Weigh 7.50g CH according to the molar ratio of Li:(Ni+Mn) of 1.47:1 3 COOLi·2H 2 O, Lithium acetate is added to the prepared nickel-manganese metal salt solution. Place the above prepared solution in a water bath at 80°C, stir magnetically until all the liquid disappears, put it in a drying oven at 80°C for 20 hours, grind the dried solid into powder, and keep it warm in an air atmosphere at 450°C for 4 hours , to obtain a lithium-rich mesophase.
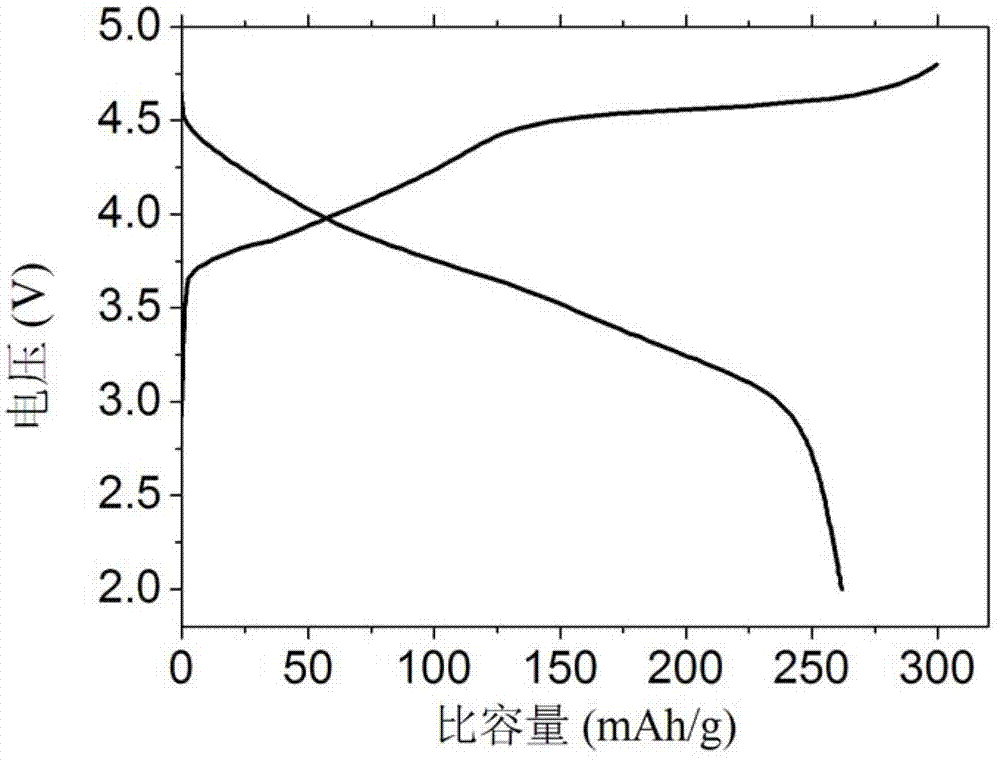
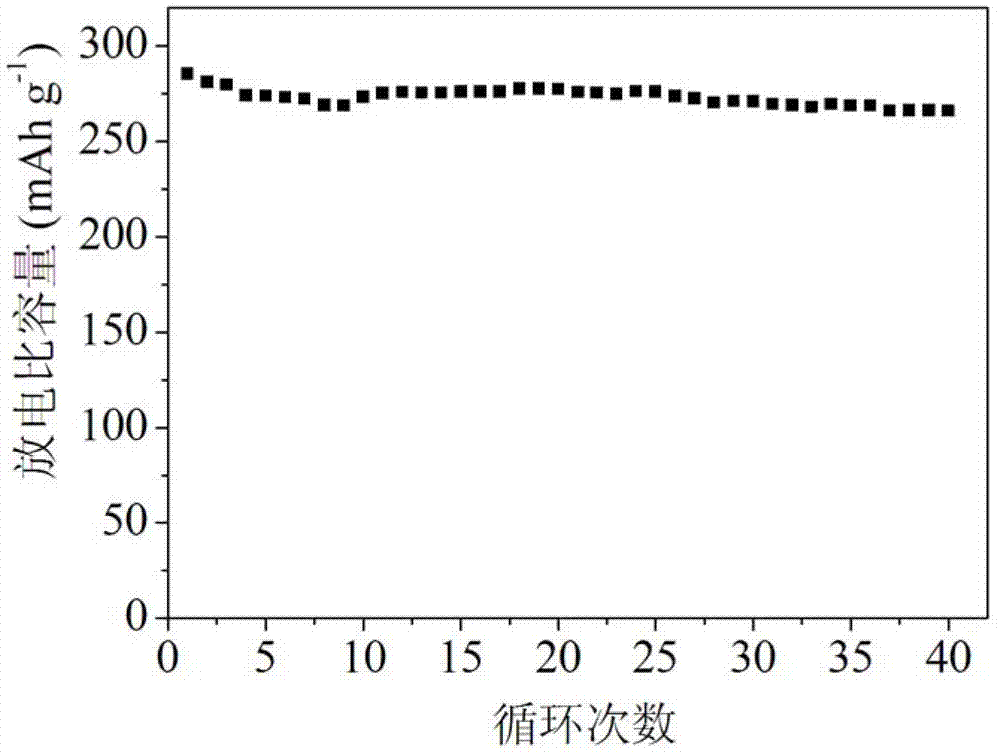
[0024] Weigh 3 g of the above lithium-rich mater...
Embodiment 2
[0029] Weigh 4.98g Ni(CH 3 COO) 2 4H 2 O and 15.00g Mn(NO 3 ) 2 4H 2 O was dissolved in 80 mL of ethanol deionized water (the volume ratio of ethanol to deionized water was 3:1), and magnetically stirred at room temperature for 2 h to fully dissolve it. Prepare ammonia water with a concentration of 0.8 mol / L, add the ammonia water dropwise to the metal salt solution, and adjust its pH value to 10.5. Take 12.04mL hydrogen peroxide and add dropwise to the above solution. Weigh 12.89g CH according to the molar ratio of Li:(Ni+Mn) of 1.58:1 3 COOLi·2H 2 O, Lithium acetate is added to the prepared nickel-manganese metal salt solution. Place the above prepared solution in a water bath at 80°C, stir magnetically until all the liquid disappears, put it in a drying oven at 80°C for 26 hours, grind the dried solid into powder, and keep it warm in an air atmosphere at 450°C for 5 hours , to obtain a lithium-rich mesophase.
[0030] Weigh 4g of the above lithium-rich material me...
Embodiment 3
[0034] Weigh 6.21g Ni(NO 3 ) 2 ·6H 2 O and 9.64g Mn(NO 3 ) 2 4H 2 O was dissolved in 100 mL of ethanol deionized water (the volume ratio of ethanol to deionized water was 3:1), and magnetically stirred at room temperature for 2 h to fully dissolve it. Prepare ammonia water with a concentration of 0.6 mol / L, add the ammonia water dropwise to the metal salt solution, and adjust its pH value to 10.5. Take 7.73mL hydrogen peroxide and add dropwise to the above solution. Weigh 3.40g LiOH·H at a molar ratio of Li:(Ni+Mn) of 1.35:1 2 O, the LiOH·H 2 O was added to the prepared nickel-manganese metal salt solution. Place the above-mentioned prepared solution in an 80°C water bath, stir magnetically until all the liquid disappears, put it into an 80°C drying oven to dry for 24 hours, grind the dried solid into a powder, and place the powder in 450°C air The atmosphere was kept warm for 6 hours to obtain a lithium-rich mesophase.
[0035] Weigh 3.5g of the above lithium-rich m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com