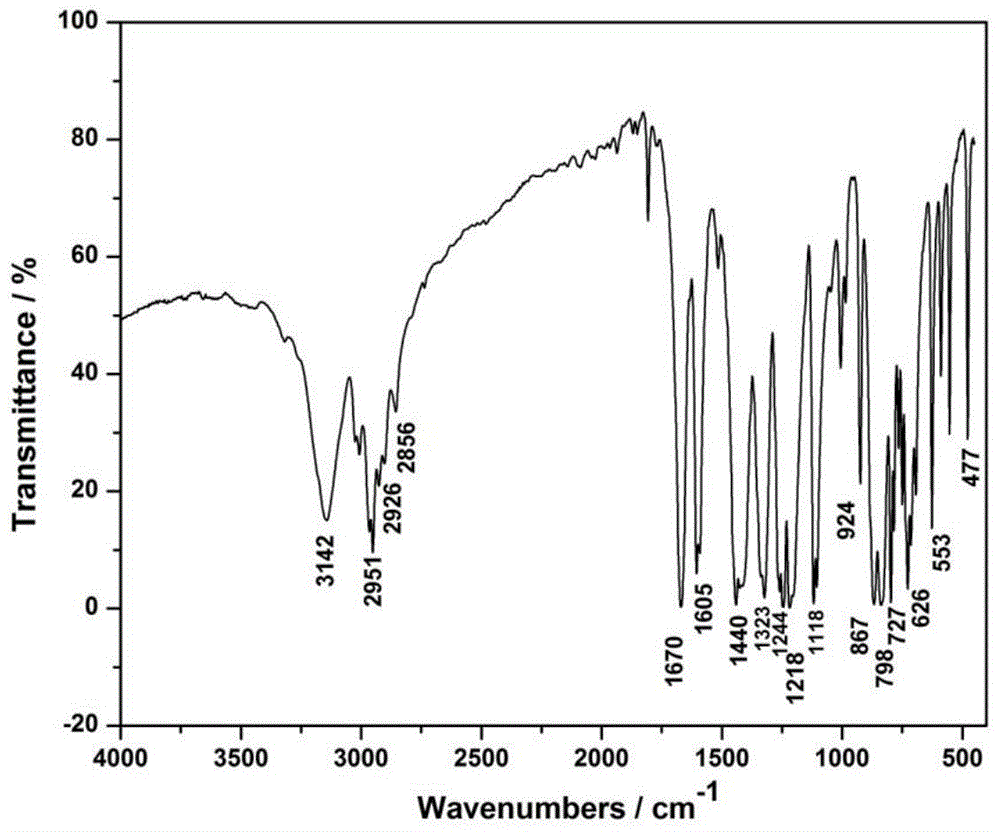
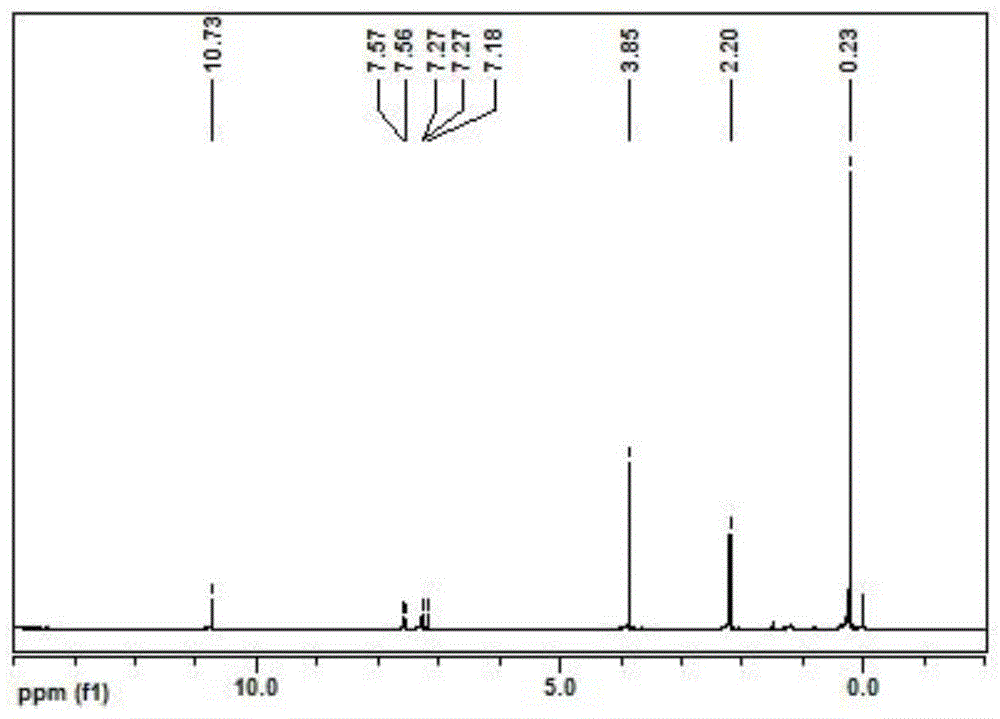
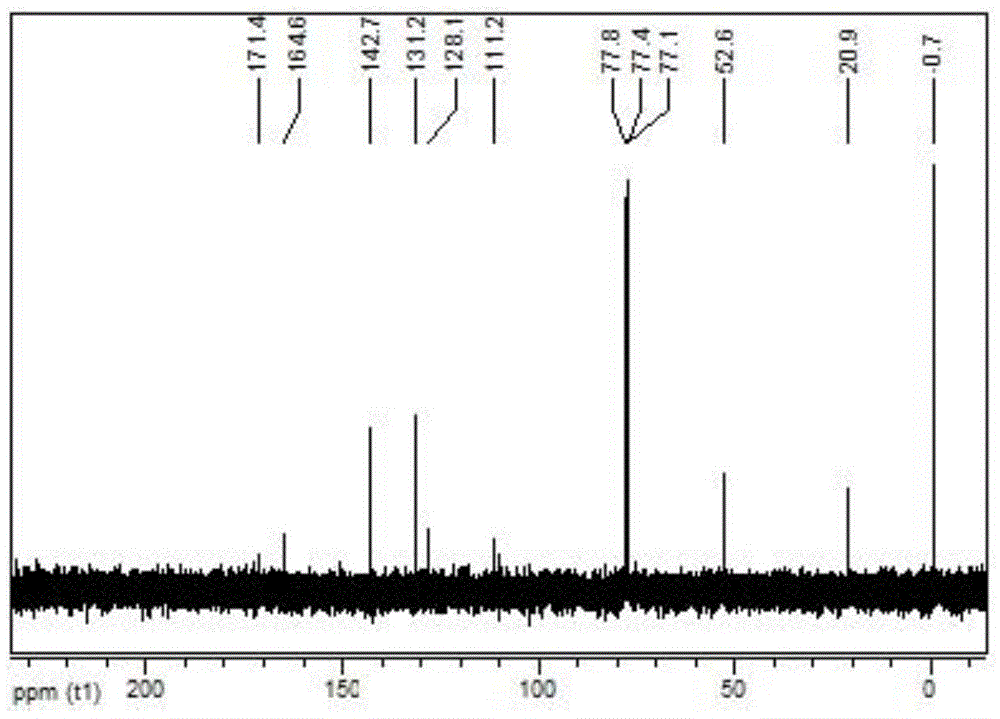
Phenoxy ester coordinated transition metal organic complex, olefin polymerization catalytic system comprising same and application of catalytic system to olefin polymerization
A technology of organic complexes and transition metals, applied in titanium organic compounds, organic chemistry, compounds of group 4/14 elements of the periodic table, etc., can solve the problem of weak ability to catalyze olefin copolymerization and achieve strong copolymerization ability , high catalytic activity and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthetic ligand L1: 5-methyl-3-trimethylsilyl salicylate methyl ester
[0046]
[0047] After drying high-purity N at high temperature 2 Add 2,6-dibromo-p-cresol (10mmol, 2.66g) and THF (10ml) into the replaced 50mL schlenk bottle, after cooling down to -20°C, add triethylamine (20.0mmol, 2.78ml) first, then Add trimethylchlorosilane (Me 3 SiCl, 15.0mmol, 1.9ml). After the dropwise addition was completed, the temperature rose to room temperature within 1 h, and the reaction was continued for 3 h. Concentrate under reduced pressure, add 20ml of ether, filter, and wash the filter residue with 10ml of ether. The filtrate was concentrated to obtain 3.3 g of a transparent colorless oily liquid, namely (2,6-dibromo-4-methylphenoxy)trimethylsilane, with a yield of 97.8%.
[0048] After high temperature baking N 2 Add (2,6-dibromo-4-methylphenoxy)trimethylsilane (1.5mmol, 0.507g) to a displaced 50ml schlenk bottle, and add Et 2 O (3.0ml). At -60°C and under good stir...
Embodiment 2
[0053] Synthesis of ligand L2: 5-methyl-3-triethylsilyl salicylate methyl ester
[0054]
[0055] After high temperature baking N 2 Add 2,6-dibromo-p-cresol (5 mmol, 1.33 g) and imidazole (5.0 mmol, 0.34 g) to a displaced 50 ml schlenk bottle, and add CH 2 Cl 2 (5ml), under good stirring conditions, the reaction system dropped to -78°C, triethylchlorosilane (20.0mmol, 3.36ml) was added instantly, the reaction system gradually rose to room temperature, and the reaction was continued for 6h. Dry the solvent in vacuo, add 100ml of n-hexane and 50ml of distilled water to the concentrated system, extract and separate, and wash the organic layer with hydrochloric acid solution (0.2N, 30ml), distilled water (30ml), saturated saline (30ml), and anhydrous MgSO 4 Dry and filter to obtain the crude product as a colorless oil. After separation by column chromatography, (2,6-dibromo-4-methylphenoxy)triethylsilane was obtained as a colorless transparent oily liquid with a yield of 99%...
Embodiment 3
[0060] Synthesis of ligand L3: methyl 5-methyl-3-tert-butyldiphenylsilyl salicylate
[0061]
[0062] After high temperature baking N 2 Add 2,6-dibromo-p-cresol (5 mmol, 1.33 g) and imidazole (5.0 mmol, 0.34 g) to a displaced 50 ml schlenk bottle, and add CH 2 Cl 2 (5ml), under good stirring conditions, the reaction system dropped to -50°C, tert-butyldiphenylchlorosilane (20.0mmol, 5.36ml) was added instantly, the reaction system gradually rose to room temperature, and the reaction was continued for 6h. Dry the solvent in vacuo, add 100ml of n-hexane and 50ml of distilled water to the concentrated system, extract and separate, and wash the organic layer with hydrochloric acid solution (0.2N, 30ml), distilled water (30ml), saturated saline (30ml), and anhydrous MgSO 4 Dry and filter to obtain the crude product as a colorless oil. Separation by column chromatography gave (2,6-dibromo-4-methylphenoxy)tert-butyldiphenylsilane as a colorless transparent crystalline solid with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com