Recombinant porcine interferon beta1-Fc fusion protein as well as encoding gene and expressing method thereof
A technology of porcine interferon and fusion protein, applied in chemical instruments and methods, methods based on microorganisms, biochemical equipment and methods, etc., can solve problems such as insolubility, lower specific activity rate of recombinant protein, and unqualified product quality, and achieve Effects of prolonging half-life, controlling preparation cost, long-acting and avoiding repeated medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 Recombinant porcine interferon beta 1-Fc fusion protein gene optimization design
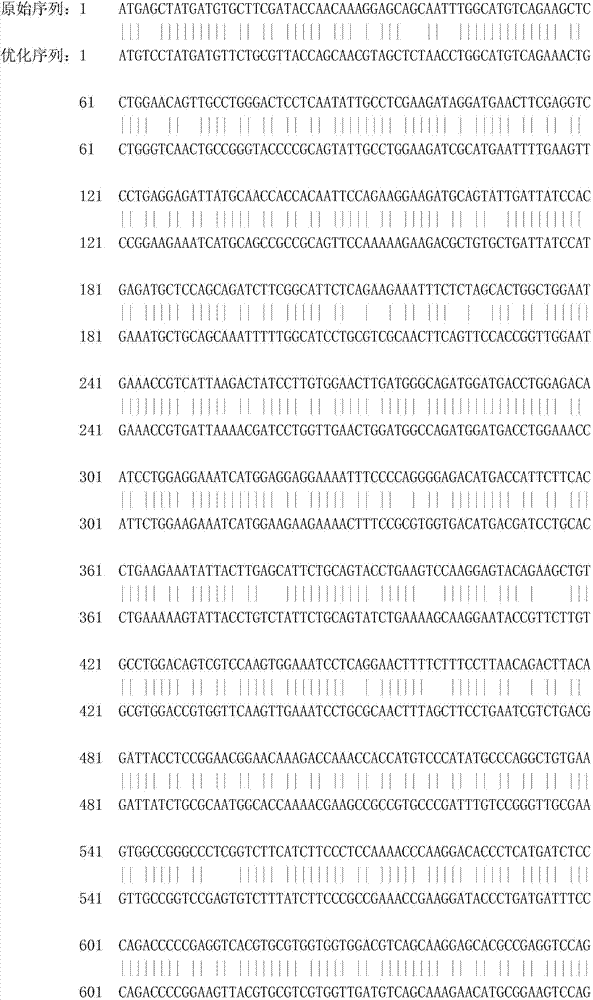
[0067]According to the cDNA sequence (GenBank accession number: NM_001003923.1) and porcine IgG Fc fragment (Sus scrofa IgG heavy chain) cDNA sequence (GenBank accession number: In the hinge region, CH2 region and CH3 region of NM_213828.1), these two genes are directly fused and codon optimized to obtain the gene of the recombinant porcine interferon β1-Fc fusion protein of the present invention, as shown in SEQ ID No: 1 .
[0068] The following is the codon optimization of the recombinant porcine interferon β1-Fc fusion protein. The parameters before and after optimization are compared as follows:
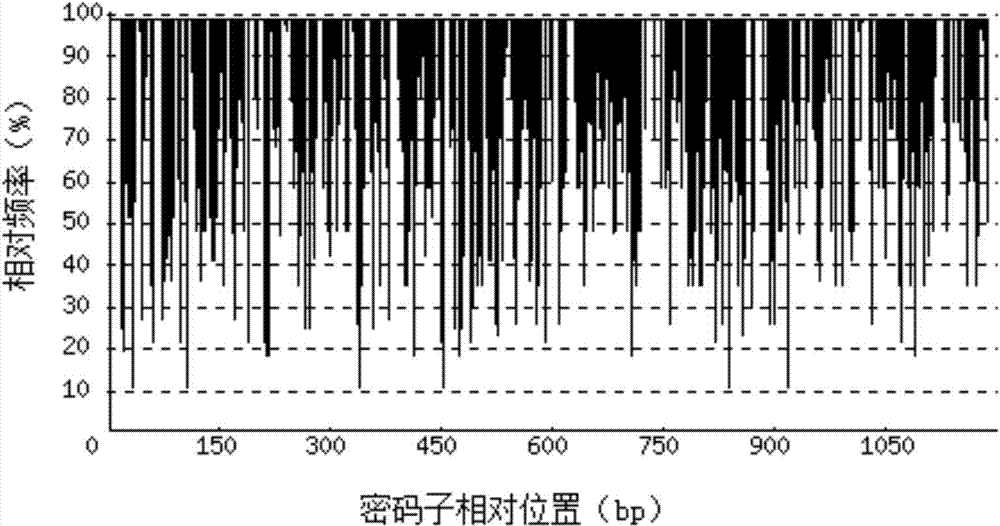
[0069] 1. Codon Adaptation Index (CAI)
[0070] Depend on Figure 2-a It can be seen that before codon optimization, the codon adaptation index (CAI) of the recombinant porcine interferon β1-Fc fusion protein gene in Escherichia coli was 0.62. Depend on Figure 2-b It can...
Embodiment 2
[0081] Embodiment 2: the expression plasmid construction of recombinant porcine interferon beta 1-Fc fusion protein gene
[0082] The fragment synthesized from the optimized recombinant porcine interferon β1-Fc fusion protein gene (as shown in SEQ ID No: 1) was constructed into the pUC57 plasmid (provided by Nanjing KingScript Co., Ltd.) to obtain a long-term Save the plasmid and call it pUC57-pIFNβ1-Fc plasmid. Using the pUC57-pIFNβ1-Fc plasmid as a template, NdeI and XhoI restriction sites were introduced upstream and downstream, respectively, for PCR amplification. The primer sequences used are as follows:
[0083] Upstream primers:
[0084] P1: CGGGAATTCCATATGATGTCCTATGATGTTCTGCG
[0085] Downstream primers:
[0086] P2: CCGCTCGAGTTATTTGCCTTGGGTCTTGC
[0087] The total volume of the reaction was 50 μL, in which 2.5 μL of each primer was added at a concentration of 10 μmol / L, and 1 μL of dNTP at a concentration of 10 mmol / L was added. The DNA polymerase used was Phusi...
Embodiment 3
[0089] Example 3 High Expression and Identification of Recombinant Porcine Interferon β1-Fc Fusion Protein in Escherichia coli
[0090] Specific steps are as follows:
[0091] 1. Transform the pET21b-pIFNβ1-Fc plasmid with correct sequencing alignment in Example 2 into Escherichia coli BL21 (DE3) competent strain (purchased from Beijing Tiangen Biochemical Technology Co., Ltd.), at 37°C, in the presence of ampicillin Plate overnight.
[0092] 2. On the next day, pick 1-4 recombinant colonies containing the pET21b-pIFNβ1-Fc plasmid, insert them into LB culture medium (purchased from Amresco) containing 100 μg / mL ampicillin, and culture overnight at 37°C.
[0093] 3. Take 50 μL of the overnight culture in step 2, add 5 mL of LB culture solution containing 100 μg / mL ampicillin, and culture with shaking at 37°C.
[0094] 4. Measure the OD of the bacterial solution every 1 hour after inoculation 600 value, to be OD 600 When =1.0, the expression was induced with 1 mmol / L IPTG ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com