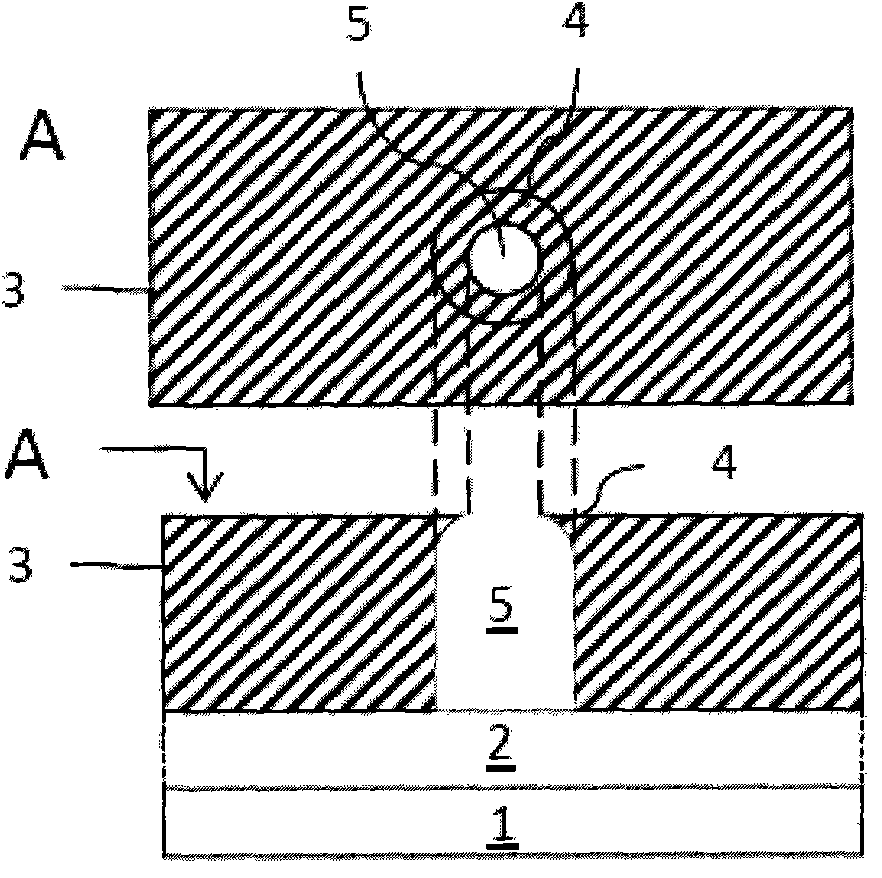
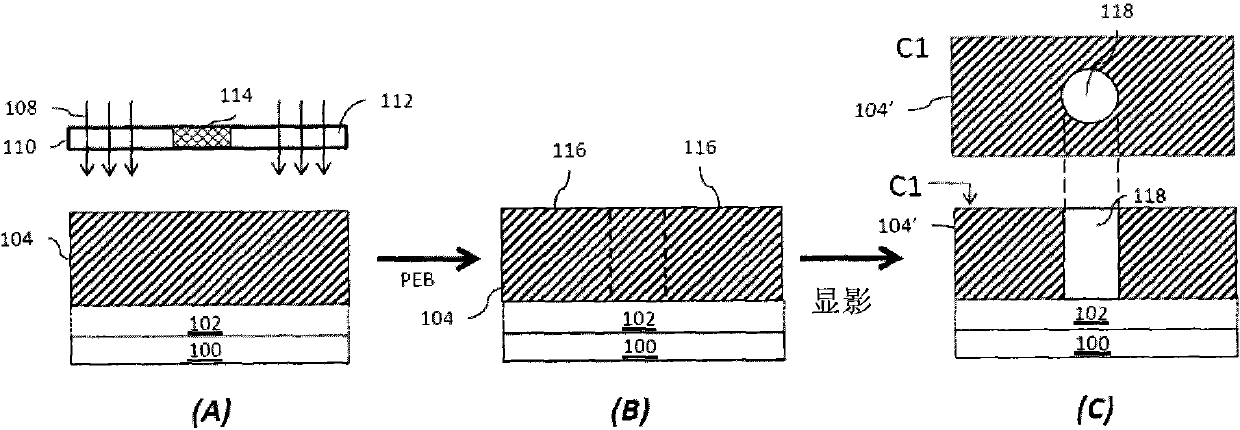
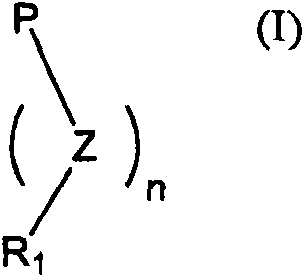Photoresist composition and method of forming photoresist pattern
A composition and photoresist technology, which can be applied to the photoengraving process of the pattern surface, the photosensitive material used in opto-mechanical equipment, optics, etc., and can solve the problems of poor processing window and affecting equipment yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1: Synthesis of Poly(ECPMA / MCPMA / MNLMA / HADA) (MP-1)
[0093] The monomers ECPMA (5.092 g), MCPMA (10.967 g), MNLMA (15.661 g) and HADA (8.280 g) were dissolved in 60 g of propylene glycol monomethyl ether acetate (PGMEA). The monomer solution was degassed by bubbling nitrogen for 20 minutes. PGMEA (27.335 g) was injected into a 500 mL three-neck flask equipped with a condenser and a mechanical stirrer, and degassed with nitrogen bubbling for 20 minutes. The solvent in the reaction flask was then warmed to 80°C. V601 (dimethyl 2,2-azobisisobutyrate) (0.858 g) was dissolved in 8 g of PGMEA, and the initiator solution was degassed with nitrogen gas bubbling for 20 minutes. The initiator solution was added to the reaction flask, and then the monomer solution was injected dropwise into the reactor, and added dropwise for 3 hours under vigorous stirring and nitrogen atmosphere. After the monomer injection was complete, the polymerization mixture was kept still at...
Embodiment 2
[0094] Example 2: Synthesis of Poly(MCPMA / OTDA / HADA)(MP-2)
[0095] Monomeric MCPMA (17.233g), OTDA (13.695g) and HADA (9.108g) were dissolved in 60g PGMEA. The monomer solution was degassed by bubbling nitrogen for 20 minutes. PGMEA (30.837 g) was injected into a 500 mL three-necked flask equipped with a condenser and a mechanical stirrer, and degassed with nitrogen bubbling for 20 minutes. The solvent in the reaction flask was then warmed to 80°C. V601 (dimethyl 2,2-azobisisobutyrate) (2.359 g) was dissolved in 8 g of PGMEA, and the initiator solution was degassed with nitrogen gas bubbling for 20 minutes. The initiator solution was added to the reaction flask, and then the monomer solution was injected dropwise into the reactor, and added dropwise for 3 hours under vigorous stirring and nitrogen atmosphere. After the monomer injection was complete, the polymerization mixture was kept still at 80° C. for an additional hour. After a total of 4 hours of polymerization ti...
Embodiment 3
[0096] Example 3: Synthesis of Poly(MCPMA / OTDA) (MP-3)
[0097]Monomeric MCPMA (17.234g) and OTDA (22.766g) were dissolved in 60g PGMEA. The monomer solution was degassed by bubbling nitrogen for 20 minutes. PGMEA (30.837 g) was injected into a 500 mL three-necked flask equipped with a condenser and a mechanical stirrer, and degassed with nitrogen bubbling for 20 minutes. The solvent in the reaction flask was then warmed to 80°C. V601 (dimethyl 2,2-azobisisobutyrate) (2.359 g) was dissolved in 8 g of PGMEA, and the initiator solution was degassed with nitrogen gas bubbling for 20 minutes. The initiator solution was added to the reaction flask, and then the monomer solution was injected dropwise into the reactor, and added dropwise for 3 hours under vigorous stirring and nitrogen atmosphere. After the monomer injection was complete, the polymerization mixture was kept still at 80° C. for an additional hour. After a total of 4 hours of polymerization time (3 hour feed and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com