Mesophilic laccase gene, mesophilic laccase and application thereof
A kind of laccase and gene technology, applied in the application field of mesophilic laccase ZGL3, textile industry and environmental protection field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
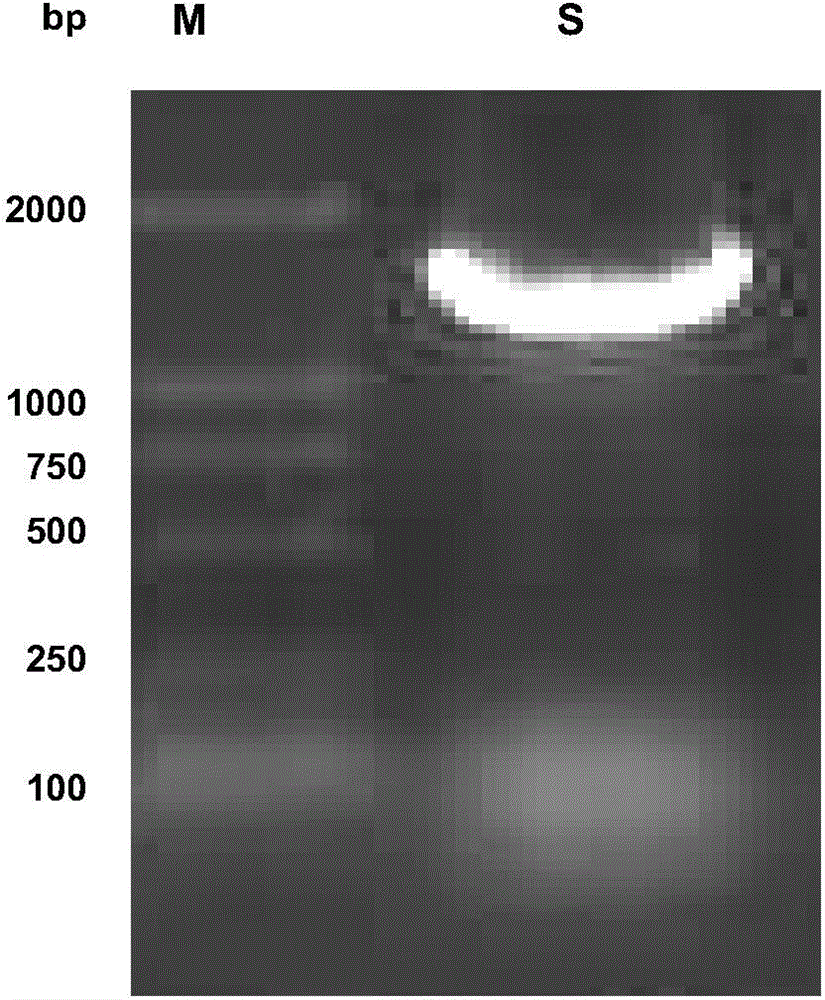
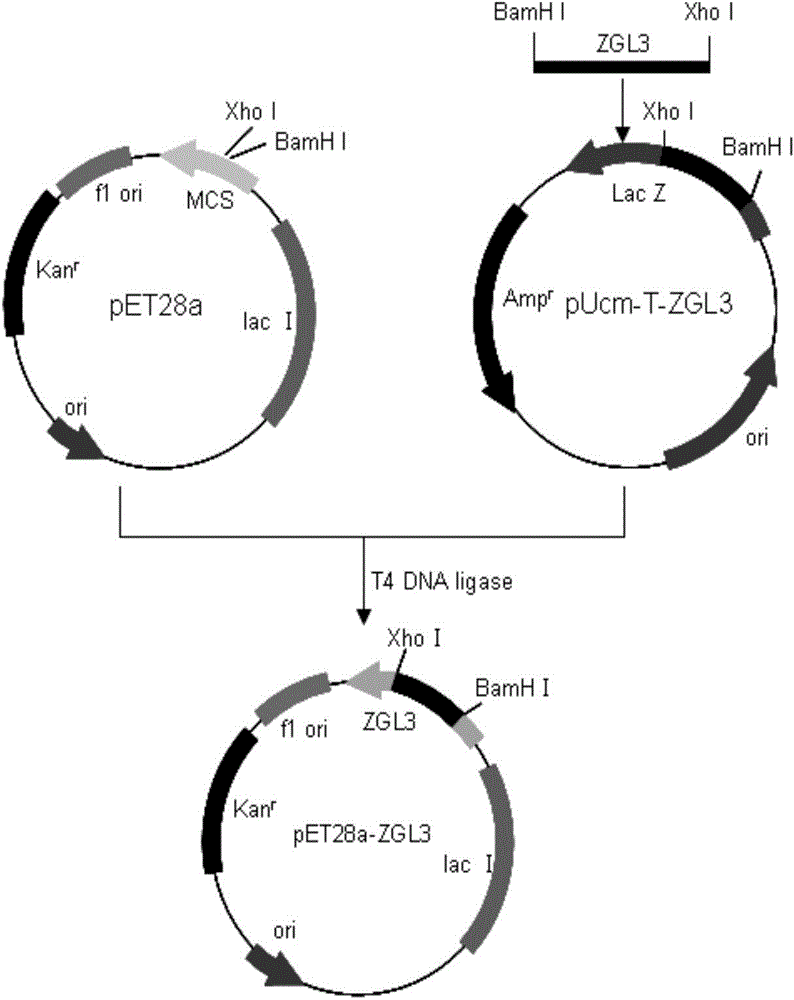
[0025] Embodiment 1: expression and preparation of mesophilic laccase ZGL3 gene cloning and mesophilic laccase ZGL3, comprising the following steps:
[0026] (1) Cultivation of mesophilic bacteria ZW2531-1 and extraction of chromosomal DNA
[0027] The medium composition of each 100ml mesophilic bacteria ZW2531-1 is as follows: yeast powder 0.4g, peptone 0.08g, NaCl 0.3g, medium pH=7.6; culture conditions are: 50°C shaker at 200rpm constant temperature culture About 8 hours; ZW2531-1 stem cells were added to 1 mL of the above-mentioned medium and cultured for 8 hours, then transferred to 100 mL of fresh above-mentioned medium for expansion for 8 hours, centrifuged at 8000 rpm for 5 minutes to collect the cultured mesophilic cells, and stored at -20°C for future use.
[0028]Take 0.2g of the mesophilic bacteria collected above, wash the bacteria once with 400μL, 25mmol / L Tris-HCl buffer (pH=8.0, containing 50mmol / L glucose, 10mmol / L EDTA), centrifuge and discard the supernatant...
Embodiment 2
[0047] Example 2: Characterization of the mesophilic laccase ZGL3
[0048] (1) Optimum reaction pH and pH stability
[0049] pH value is an important factor affecting the catalytic activity of enzymes. Usually the enzyme shows the maximum catalytic activity at a certain pH value, and the farther it deviates from this pH value, the lower the catalytic activity of the enzyme. The maximum pH range detected by the catalytic activity of the mesophilic laccase ZGL3 of the present invention is 2.2~ 10.0.
[0050] Using ABTS and DMP as substrates, measure the activity of mesophilic laccase ZGL3 in buffers with different pH values (2.2.0-10.0), and calculate the relative enzyme activity under other pH conditions with the highest enzyme activity as 100%. . The relative activity of the enzyme is plotted against the pH value ( Figure 5 A), the results show that the optimum pH values of ABTS and DMP catalyzed by mesophilic laccase ZGL3 are 4.0 and 7.5, respectively.
[0051] After ...
Embodiment 3
[0056] Example 3: Efficacy Analysis of Mesophilic Laccase ZGL3 Catalytic Dye Decolorization
[0057] Using ABTS and syringaldehyde as mediator substrates respectively, the mesophilic laccase ZGL3 was analyzed for five dyes reactive blue 19 (Remazol Brilliant Blue R, RBBR), Congo red (Congo red), indigo carmine (indigo carmine), formazan The decolorization efficiency of different concentrations of base red (MethyI red) and crystal violet (Crystal violet) (100mg / L, 10mg / L, 40mg / L, 50mg / L, 5mg / L), the decolorization rate is calculated by the following formula: decolorization Rate% = (initial absorbance value - absorbance value after catalysis) / initial absorbance value × 100%.
[0058] Table 1. Efficacy analysis of dye decolorization catalyzed by mesophilic laccase ZGL3
[0059]
[0060] The results are shown in Table 1, Figure 7 shown. In the absence of mediator substrates, mesophilic laccase ZGL3 can effectively decolorize RBBR, Congo red and indigo carmine, but is less...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com