Catalyst for CO hydrogenation reaction, preparation method and application thereof
A catalyst and oxide technology, which is applied to the catalyst for preparing high-carbon hydrocarbons and the field of preparation thereof, can solve the problems of increasing workload and energy consumption such as gas circulation compression, and achieve the effect of high single-pass conversion rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
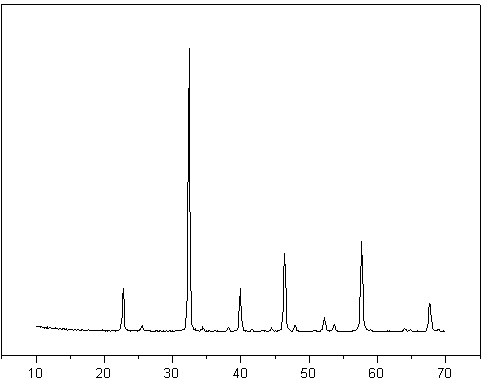
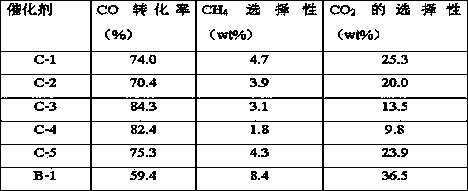
[0021] Prepare a mixed aqueous solution containing ferric nitrate and lanthanum nitrate, weigh an appropriate amount of citric acid according to the molar ratio of citric acid to the total amount of metal ions in the mixed aqueous solution is 1.2:1, slowly add citric acid to the mixed aqueous solution, and stir while adding. After stirring for 5 hours, the brown solution had been dehydrated and turned into a viscous gel. The gel was taken out and placed in a drying oven at 110° C. to dry overnight. Then take out the dried precursor, place it in a muffle furnace and roast at a constant temperature of 800 °C for 4 hours to obtain a composite metal oxide LaFeO with a perovskite structure. 3-y , using the impregnation method in the composite metal oxide LaFeO 3-y The additive potassium with a weight content of 5% was loaded on it, dried at 80°C for 8 hours, and calcined at 350°C for 4 hours to obtain a catalyst which was designated as C-1. The evaluation results are shown in Table...
Embodiment 2
[0023] Prepare a mixed aqueous solution containing ferric nitrate, cerium nitrate and nickel nitrate, weigh an appropriate amount of citric acid according to the molar ratio of citric acid to the total amount of metal ions in the mixed aqueous solution is 2:1, slowly add citric acid to the mixed aqueous solution, and add While stirring. After stirring for 5 hours, the brown solution had been dehydrated and turned into a viscous gel. The gel was taken out and placed in a drying oven at 110° C. to dry overnight. Then take out the dried precursor, place it in a muffle furnace and roast at a constant temperature of 700 °C for 6 hours to obtain a composite metal oxide CeFe with a perovskite structure. 0.9 Ni 0.1 o 3-y , using the impregnation method on the composite metal oxide CeFe 0.9 Ni 0.1 o 3-y Zinc with a weight content of 10% was loaded on it, and the prepared catalyst was recorded as C-2, and the evaluation results are shown in Table 1.
Embodiment 3
[0025] Prepare a mixed aqueous solution containing neodymium nitrate, iron nitrate and nickel nitrate, weigh an appropriate amount of citric acid according to the molar ratio of citric acid to the total amount of metal ions in the mixed aqueous solution is 2:1, slowly add citric acid to the mixed aqueous solution, and add While stirring. After stirring for 5 hours, the brown solution had been dehydrated and turned into a viscous gel. The gel was taken out and placed in a drying oven at 110° C. to dry overnight. Then take out the dried precursor, place it in a muffle furnace and roast at a constant temperature of 700 °C for 6 hours to obtain a composite metal oxide NdFe with a perovskite structure. 0.9 Ni 0.1 o 3-y , using the impregnation method in the composite metal oxide NdFe 0.9 Ni 0.1 o 3-y Zinc with a weight content of 10% was loaded on it, and the prepared catalyst was recorded as C-3, and the evaluation results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com