Tea polysaccharide derivative and preparation method thereof
A technology for tea polysaccharides and derivatives, which is applied in the field of tea polysaccharide derivatives and their preparation, can solve problems such as the preparation methods of tea polysaccharide derivatives that have not yet been seen, and achieve the effects of cheap equipment and raw materials, convenient operation, and maintaining biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
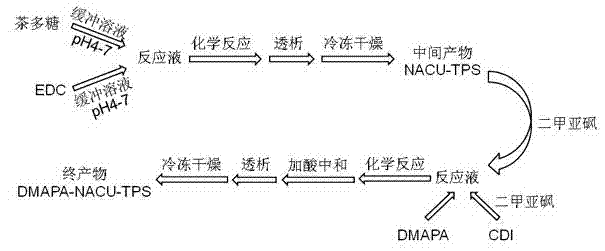
[0039] (1) Weigh 0.5 g of tea polysaccharide, put it in 60 ml of acetic acid-sodium acetate buffer solution (pH 4.0), and stir at 20°C for 20 hours to dissolve.
[0040] (2) Weigh 5 g of EDC, put it in 8 ml of acetic acid-sodium acetate buffer (pH 4.0), stir at 20°C for 8 minutes to dissolve.
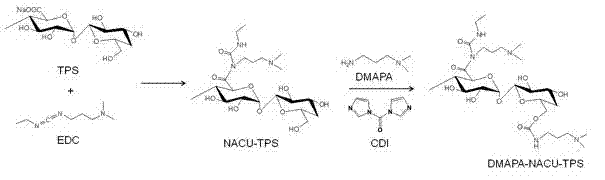
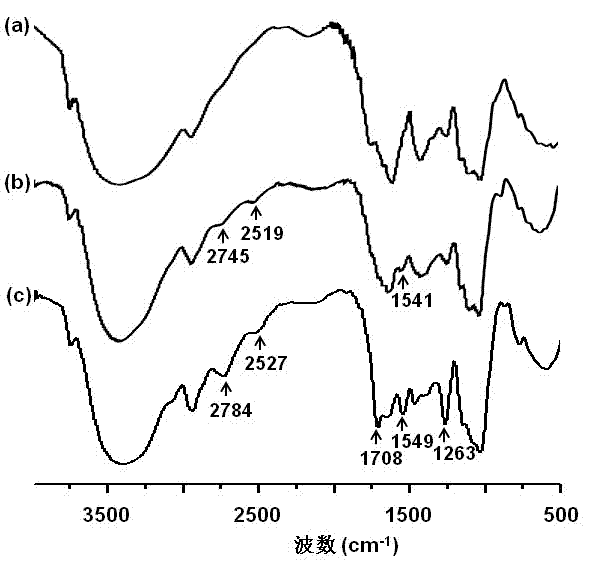
[0041] (3) Add the mixed solution obtained in step (2) dropwise to the mixed solution obtained in step (1), react at 20°C for 10 hours, put the reaction solution into a dialysis bag (molecular weight cut-off 1,000), and dialyze against distilled water for 3 days , and the intermediate product was obtained after freeze-drying, which was named NACU-TPS. The structural analysis was carried out by infrared spectroscopy and nuclear magnetic resonance spectroscopy experiments, and the degree of substitution was calculated to be 0.3.
[0042] (4) Weigh 0.3 g of the intermediate product NACU-TPS obtained in step (3), dissolve it in 30 ml of anhydrous dimethyl sulfoxide, and stir at 20°C for 24 ...
Embodiment 2
[0052] (1) Weigh 1 gram of tea polysaccharide, put it in 100 ml of citric acid-sodium citrate buffer (pH 4.4), stir at 25°C for 10 hours to dissolve.
[0053] (2) Weigh 3 g of EDC, put it in 5 ml of citric acid-sodium citrate buffer (pH 4.4), and stir at 25°C for 20 minutes to dissolve.
[0054] (3) Add the mixed solution obtained in step (2) dropwise to the mixed solution obtained in step (1), react at 25°C for 20 hours, put the reaction solution into a dialysis bag (molecular weight cut-off 500), and dialyze against distilled water for 2 days , and the intermediate product was obtained after freeze-drying, which was named NACU-TPS. The structural analysis was carried out by infrared spectroscopy and nuclear magnetic resonance spectroscopy experiments, and the degree of substitution was calculated to be 0.3.
[0055] (4) Weigh 0.5 g of the intermediate product NACU-TPS obtained in step (3), dissolve it in 25 ml of anhydrous dimethyl sulfoxide, and stir at 25°C for 10 hours to...
Embodiment 3
[0059] (1) Weigh 0.1 g of tea polysaccharide, put it in 10 ml of disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution (pH5.8), stir at 15°C for 24 hours to dissolve.
[0060] (2) Weigh 1 gram of EDC, put it in 3 ml of disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution (pH5.8), and stir at 15°C for 30 minutes to dissolve.
[0061] (3) Add the mixed solution obtained in step (2) dropwise to the mixed solution obtained in step (1), react at 15°C for 24 hours, put the reaction solution into a dialysis bag (molecular weight cut-off 2,000), and dialyze against distilled water for 5 days , and the intermediate product was obtained after freeze-drying, which was named NACU-TPS. The structural analysis was carried out by infrared spectroscopy and nuclear magnetic resonance spectroscopy experiments, and the degree of substitution was calculated to be 0.1.
[0062] (4) Weigh 0.1 g of the intermediate product NACU-TPS obtained in step (3), dissolve ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com