Long-acting compound sulfametoxydiazine sodium injection for veterinary use and preparation method thereof
A technology for sodium sulfa-p-methoxy-pyrimidine and sodium methoxy-pyrimidine is applied in the field of veterinary long-acting compound sulfa-p-methoxy-pyrimidine sodium injection and preparation thereof, and can solve the problem of vitamin B1 deficiency, high irritation, and high liver and kidney toxicity. and other problems, to achieve the effect of reducing toxic side effects, reducing irritation, and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
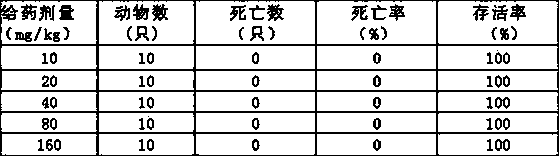
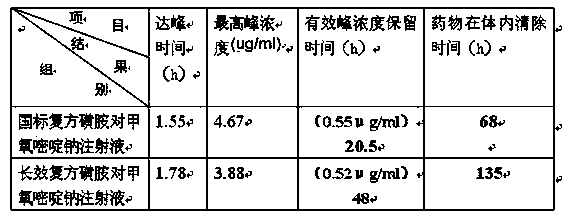
Image
Examples
Embodiment 1
[0032] (1) Weigh 50g of glycerol methylal, 100g of α-pyrrolidone, and 200g of polyethylene glycol 400 (PEG400) into the batching tank, heat to 70-80°C, add 150g of polyvinylpyrrolidone while stirring, and then stir while stirring. Add trimethoprim 20g, continue to stir for 10-20 minutes to dissolve, for subsequent use;
[0033] (2) Weigh 10-20g of water for injection and put it into a stainless steel bucket and heat it to 60-80℃, add sulfonamide to
[0034] 10 g of sodium methoxine was stirred and dissolved, for use;
[0035] (3) Weigh 10-20g of water for injection and put it into a stainless steel bucket and heat it to 60-80℃, add 30g of taurine and 50g of vitamin B1, stir and dissolve, and set aside for later use;
[0036] (4) Add solution (2) and solution (3) to solution (1) in turn, stir evenly, and add 5 g of benzyl alcohol. Adjust the pH to 7.0-8.0 with 10g of sodium butyrate or 10g of arginine, and add water for injection to 1000ml;
[0037] (5) After filtration, fil...
Embodiment 2
[0039] (1) Weigh 100 g of glycerol methylal, 150 g of α-pyrrolidone, and 300 g of polyethylene glycol 400 (PEG400) into the batching tank, heat to 70-80 °C, add 200 g of polyvinylpyrrolidone while stirring, and then stir while stirring. Add trimethoprim 40g, continue to stir for 10-20 minutes to dissolve, for subsequent use;
[0040] (2) Weigh 10-20g of water for injection and put it into a stainless steel bucket and heat it to 60-80°C, add 20g of sulfamethoxine sodium and stir to dissolve, for later use;
[0041] (3) Weigh 10-20g of water for injection and put it into a stainless steel bucket and heat it to 60-80℃, add 50g of taurine and 100g of vitamin B1, stir and dissolve, and set aside for later use;
[0042] (4) Add solution (2) and solution (3) to solution (1) in turn, stir evenly, and add 10 g of benzyl alcohol. Adjust the pH to 7.0-8.0 with 20g of sodium butyrate or 20g of arginine, and add water for injection to 1000ml;
[0043] (5) After filtration, fill in ampoul...
Embodiment 3
[0045] (1) Weigh 80 g of glycerol methylal, 120 g of α-pyrrolidone, and 230 g of polyethylene glycol 400 (PEG400) into the batching tank, heat to 70-80 ° C, add 200 g of polyvinylpyrrolidone while stirring, and then stir Add trimethoprim 20g, continue to stir for 10-20 minutes to dissolve, for subsequent use;
[0046] (2) Weigh 10-20g of water for injection and put it into a stainless steel bucket and heat it to 60-80℃, add 10g of sulfamethoxine sodium, stir and dissolve, and set aside for later use;
[0047] (3) Weigh 10-20g of water for injection and put it into a stainless steel bucket and heat it to 60-80℃, add 40g of taurine and 70g of vitamin B1, stir and dissolve, and set aside for later use;
[0048] (4) Add solution (2) and solution (3) to solution (1) in turn, stir evenly, and add 7g of benzyl alcohol. Adjust the pH to 7.0-8.0 with 10g of sodium butyrate or 10g of arginine, and add water for injection to 1000ml;
[0049] (5) After filtration, fill in ampoules with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com