Method for reducing energy consumption in preparing sodium nitrite through nitrogen oxide
A technology of sodium nitrite and nitrogen oxides, applied in the directions of nitrogen oxides/oxyacids, nitrous acid, etc., can solve problems such as being unsuitable for industrial production, and achieve no influence on the quality of sodium nitrite, easy to popularize and reduce The effect of steam consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
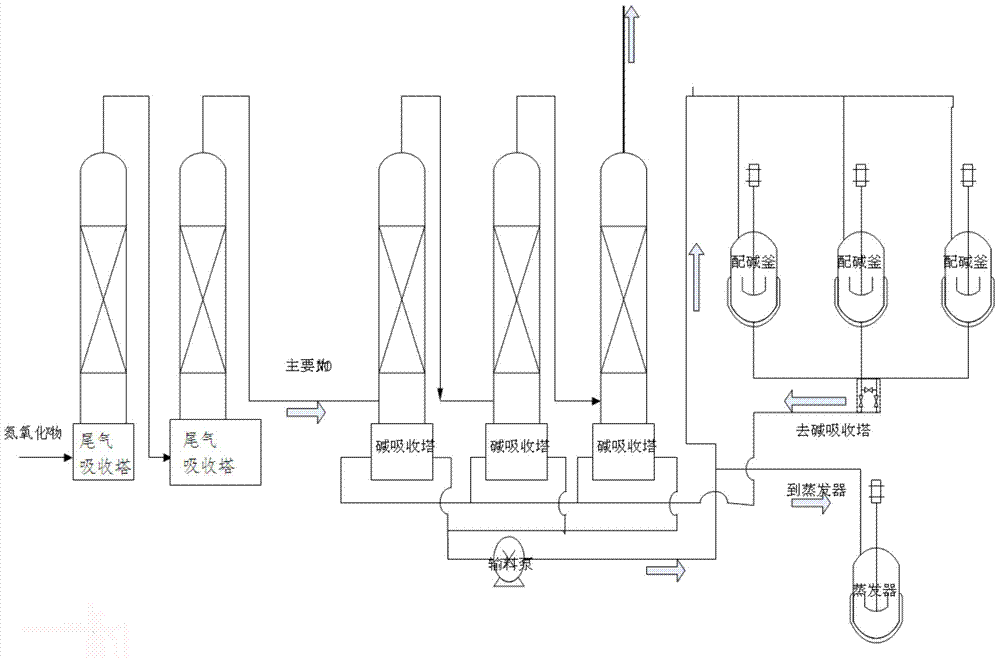
[0044] A method for reducing nitrogen oxides to prepare sodium nitrite energy consumption, comprising the steps of:
[0045] (1) Prepare a soda ash solution with a mass percentage concentration of 10% to 25% in a primary alkali preparation kettle under the condition of 30-40°C (primary alkali preparation), and set aside;
[0046] (2) After the nitrogen oxides produced in the factory are collected, they are introduced into the tail gas absorption tower for treatment. The tail gas absorption tower uses water as the medium to absorb nitrogen oxides and remove most of the NO 2 , the remaining NO, NO 2 Introduce the alkali absorption tower, and the tail gas absorption tower is generally set at 2 to 4 levels;
[0047] (3) Put the soda ash solution prepared in step (1) into the alkali absorption tower, and introduce the tail gas treated in the tail gas absorption tower in step (2) into the alkali absorption tower for reaction to generate sodium nitrite. The chemical reaction formula...
Embodiment 2
[0056] 1. Under the condition of 30-40℃, add 150kg of anhydrous sodium carbonate (that is, soda ash) under stirring in a primary alkali mixing tank with 1000L water, and prepare about 13% soda ash solution (primary mixing alkali), and set aside;
[0057] 2. Put the prepared soda ash solution into the two-stage alkali absorption tower, introduce the tail gas treated in the tail gas absorption tower, and react at room temperature (25°C);
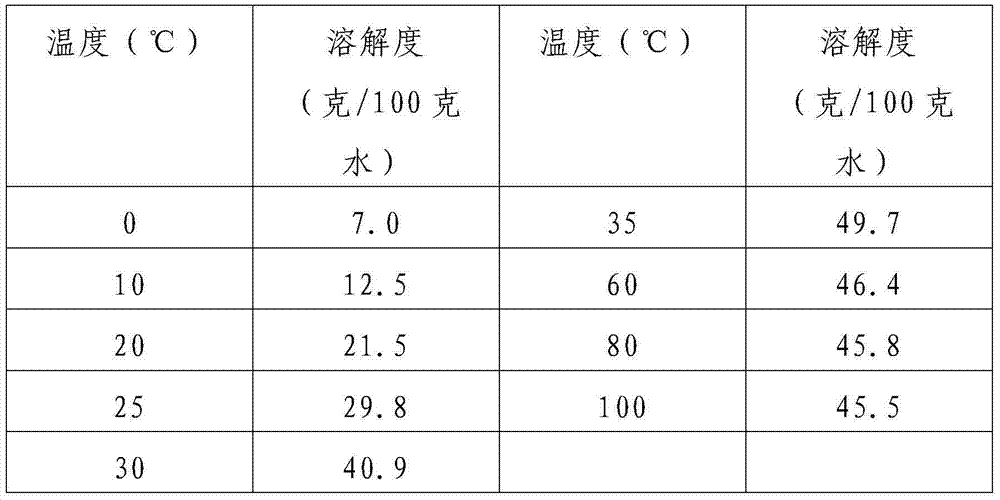
[0058] 3. When the pH value of the solution is 6-7, the reaction reaches the end point, and the resulting sodium nitrite solution is transported to the concentration kettle, the remaining water is evaporated, the temperature is lowered and crystallized, and centrifuged to obtain 155kg, 98% or more of nitrous acid sodium products.
[0059] According to statistics, about 6.0 tons of steam are consumed per ton of sodium nitrite.
Embodiment 3
[0061] 1. Under the condition of 30-40°C, add 300kg of anhydrous sodium carbonate (that is, soda ash) under stirring in a primary caustic soda mixing tank with 2000L water, and prepare about 13% soda ash solution (primary soda ash mixing), and set aside;
[0062] 2. Put the prepared soda ash solution into the two-stage alkali absorption tower, introduce the tail gas treated in the tail gas absorption tower, and react at room temperature (25°C);
[0063] 3. When the pH value of the solution is 6-7, the reaction reaches the end point, and the generated sodium nitrite solution is retransmitted to the secondary alkali preparation kettle, the temperature is raised to 30-40 °C, and 300 kg of anhydrous sodium carbonate ( i.e. soda ash), prepared into a secondary soda ash solution containing sodium nitrite;
[0064] 4. Put the secondary soda ash solution back into the alkali absorption tower, continue to introduce the tail gas treated by the tail gas absorption tower, and react at roo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com