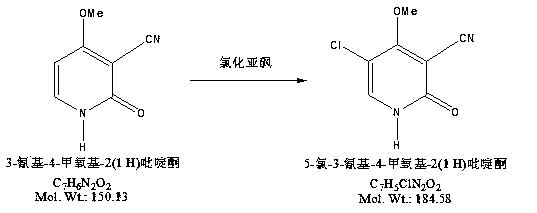
Synthesis method of 5-chloro-3-cyano-4-methony-2-(1H)-pyridinone
A synthetic method, methoxyl technology, applied in the direction of organic chemistry, can solve the problems of low product purity, difficulty in purification, incomplete reaction of raw materials, etc., and achieve the effect of high product purity and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Ingredients:
[0017] ①3-cyano-4-methoxy-2(1 H)pyridone 20g
[0018] ②thionyl chloride 100ml
[0019] Operation: Slowly drop thionyl chloride into 3-cyano-4-methoxy-2(1 H)pyridone at a temperature of 0-5°C, keep the temperature below 5°C, drop thionyl chloride Accelerate at 1.5 ml / min, then slowly raise the temperature until reflux occurs, keep the reaction for 6 hours, evaporate the thionyl chloride to dryness under reduced pressure, add 100ml of n-hexane to beat the solid for 1 hour, filter, and dry to obtain the product. The yield is 92.7%.
[0020] The density of the obtained product: 1.434g / cm3, the boiling point under the pressure of 760 mmHg is: 376.845°C, and the flash point is 181.709°C.
Embodiment 2
[0022] Ingredients:
[0023] ①3-cyano-4-methoxy-2(1 H)pyridone 20g
[0024] ②thionyl chloride 100ml
[0025] Operation: Slowly drop thionyl chloride into 3-cyano-4-methoxy-2(1 H)pyridone at a temperature of 0-5°C, keep the temperature below 5°C, drop thionyl chloride The acceleration is 1.2ml / min, and then the temperature is raised slowly until reflux occurs, and the reaction is kept for 6.5 hours. The thionyl chloride is evaporated to dryness under reduced pressure, and 100ml of n-hexane is added to beat the solid for 1 hour, filtered, and dried to obtain the product. The yield is 91.4%.
[0026] Density of the resulting product: 1.432g / cm 3, , The boiling point at 760 mmHg pressure is: 376.795°C, and the flash point is 181.694°C.
Embodiment 3
[0028] Ingredients:
[0029] ①3-cyano-4-methoxy-2(1 H)pyridone 20g
[0030] ②thionyl chloride 100ml
[0031] Operation: Slowly drop thionyl chloride into 3-cyano-4-methoxy-2(1 H)pyridone at a temperature of 0-5°C, keep the temperature below 5°C, drop thionyl chloride Accelerate at 2.0ml / min, then slowly raise the temperature until reflux occurs, keep the reaction for 5.5 hours, evaporate the thionyl chloride to dryness under reduced pressure, add 100ml of n-hexane to beat the solid for 1 hour, filter, and dry to obtain the product. The yield is 92.7%.
[0032] Density of the resulting product: 1.438g / cm 3, , The boiling point at 760 mmHg pressure is: 376.892°C, and the flash point is 181.796°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com