Method of preparing Alogliptin
A compound and reaction technology, applied in the field of preparation of alogliptin and its benzoate and its intermediates, can solve problems such as relatively expensive starting raw materials, unfavorable industrial production, and no obvious cost advantage, so as to avoid Use of high boiling point solvents and highly dangerous reagents, simplified operation, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
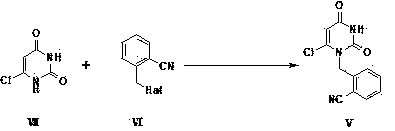
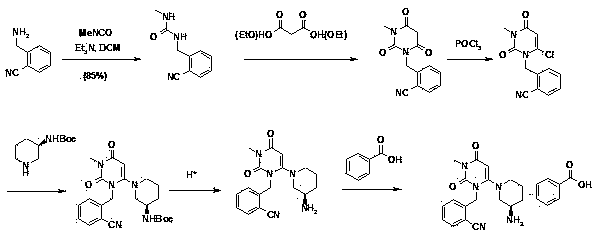
[0059] Example 1 Preparation of 2-(6-chloro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile (Ⅴ)
[0060] 1a) Suspend 6-chlorouracil (50g) in tetrahydrofuran (300ml), add N,N-diisopropylethylamine (80ml), then dropwise add 2-cyanobenzyl bromide (66.3g) Tetrahydrofuran (100ml) solution. The mixed solution was stirred and reacted at 20-65°C, a large amount of solids precipitated out, and the reaction was detected by thin-layer chromatography. After the reaction was complete, the solid formed by the reaction was filtered, washed with tetrahydrofuran (30ml), and then poured into 100ml of water and stirred for 5 minutes. After filtering, washing with water and drying under reduced pressure, 50 g of 2-(6-chloro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile (Ⅴ) was obtained. The previous tetrahydrofuran mother liquor was concentrated and then recrystallized with tetrahydrofuran to obtain another 15 g of the target product, with a total yield of 72.8%.
[00...
Embodiment 2
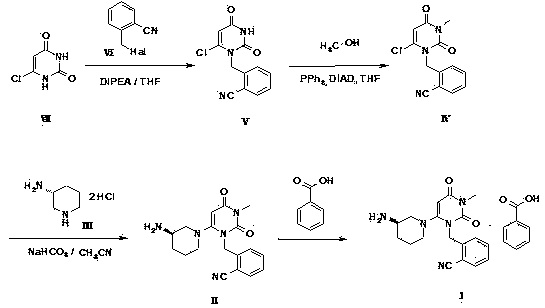
[0062] Example 2 Preparation of 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)benzonitrile (IV)
[0063] 2a) Suspend compound V (5.22g) in tetrahydrofuran (40ml), add methanol (3ml) and triphenylphosphine (7.77g) in sequence, add diisopropyl azodicarboxylate dropwise to the reaction solution at room temperature Ester (6.02g), the mixture was reacted at room temperature for 3 hours, and the reaction was complete as detected by thin layer chromatography. The reaction solution was concentrated under reduced pressure, and the residue was recrystallized from a mixed solution of methanol and methyl tert-butyl ether to obtain 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro -2H-pyrimidin-1-ylmethyl)benzonitrile (IV) 4g, the mother liquor was concentrated and subjected to silica gel column chromatography (gradient elution with petroleum ether-ethyl acetate as the eluent) to obtain 1.2g of the target product, The total reaction yield is 94.5%.
[0064] 2b) Suspend comp...
Embodiment 3
[0066] Example 3 2-{6-[3(R)-amino-piperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidine-1- Preparation of methyl}-benzonitrile benzoate (Ⅰ)
[0067] 3a) Compound IV (5.2g), sodium bicarbonate (4.2g) and (R)-3-amino-piperidine dihydrochloride (3.48g) were suspended in acetonitrile (200ml), and refluxed for 14 hours. Thin-layer chromatography detected that the reaction was complete, the reaction solution was lowered to room temperature, filtered to remove most of the sodium bicarbonate, the mother liquor was concentrated, the residue was dissolved in dichloromethane, washed with water, dried over anhydrous sodium sulfate for half an hour, and filtered to remove sodium sulfate. Add benzoic acid (4 g) to the filtrate, stir at room temperature for 2 hours, concentrate under reduced pressure, dissolve the residue with a small amount of methanol, and then add diethyl ether or methyl tert-butyl ether to precipitate an off-white solid. The white solid was filtered off and recry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com