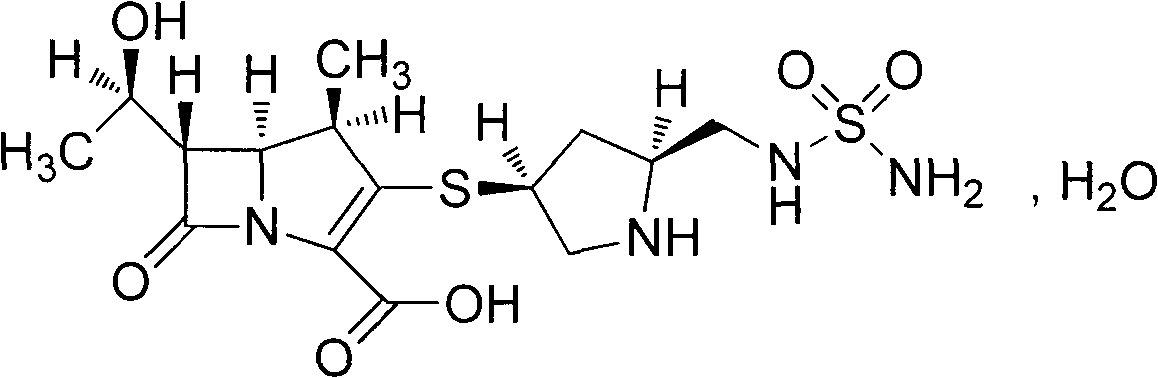
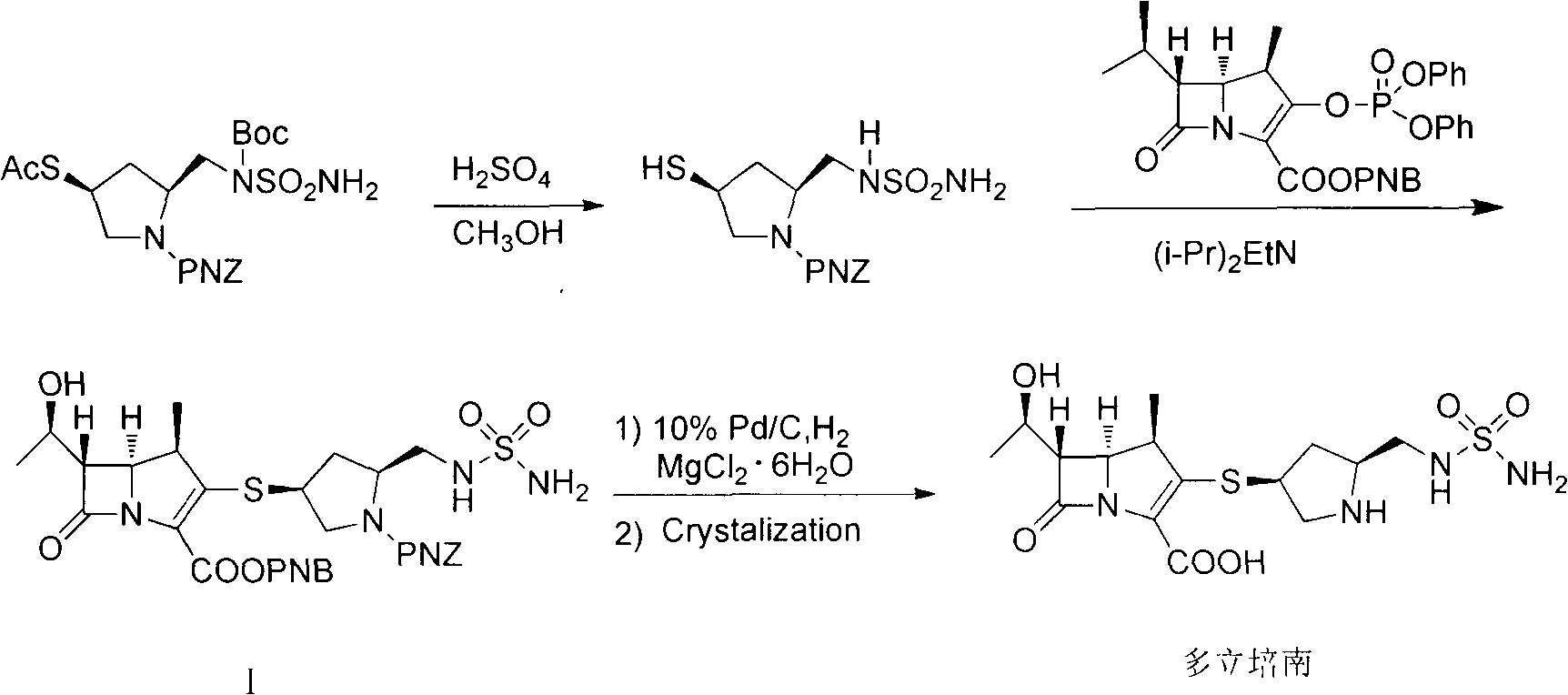
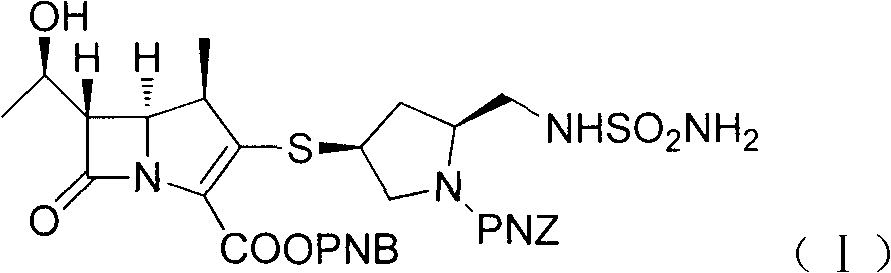
Industrial synthesis method of doripenem
A technology of doripenem and compound, applied in the field of industrialized preparation of doripenem, can solve the problems of many organic solvents, low product purity, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 150g of intermediate (I) to the hydrogenation kettle, dissolve it with 1000ml of ethyl acetate, add 750ml of saturated sodium carbonate solution (pH10), 75g of 10% Pd / C, seal the kettle body, replace the kettle with nitrogen Air, repeated three times, after vacuuming, hydrogen gas was introduced, the pressure in the kettle was kept at 0.6MPa, the temperature was kept at about 40°C, and the reaction was carried out for 4h. Filter, wash the filter cake with 100ml of saturated sodium carbonate solution, separate the filtrate, wash the water phase with 300ml of ethyl acetate, cool the water phase to below 10°C with an ice-water bath, adjust the pH to 5 with 2N hydrochloric acid, and gradually precipitate solids at 10°C Stir for 2 hours, filter, wash the filter cake with 150 ml of 80% isopropanol, and vacuum-dry at 50°C (gauge pressure not less than 0.095 MPa) for about 6 hours to obtain 73.7 g of white crystalline powder, HPLC purity 99.0%, yield 82.3% .
Embodiment 2
[0044] Add 150g of intermediate (I) to the hydrogenation kettle, add 1500ml of n-butanol and stir, add 750ml of saturated sodium bicarbonate solution (pH 8.4), 75g of 10% Pd / C, seal the kettle body, and replace it with nitrogen gas The air in the kettle was removed, repeated three times, and hydrogen gas was introduced after vacuuming. The pressure in the kettle was kept at 0.6 MPa, the temperature was kept at about 40°C, and the reaction was carried out for 4 hours. Filter, wash the filter cake with 100ml of saturated sodium bicarbonate solution, separate the layers of the filtrate, wash the water phase with 300ml of ethyl acetate, cool the water phase to below 10°C with an ice-water bath, adjust to pH 5 with 2N hydrochloric acid, and gradually precipitate solids, 10 Stir for 2 hours below ℃, filter, wash the filter cake with 150ml of 80% isopropanol, and vacuum-dry at 50℃ (gauge pressure not lower than 0.095MPa) for about 6 hours to obtain 63.1g of off-white crystalline powde...
Embodiment 3
[0046] Take 54g of ammonium chloride, add 200ml of water to dissolve it, add 350ml of concentrated ammonia solution, and then dilute to 1000ml with water to obtain an ammonia-ammonium chloride buffer solution with a pH of 10.
[0047]In the hydrogenation kettle, add intermediate (I) 150g, add ethyl acetate 1000ml and stir, add ammonia-ammonium chloride buffer solution 750ml, 10% Pd / C 75g, after sealing the kettle body, first replace the kettle with nitrogen Air, repeated three times, after vacuuming, hydrogen gas was introduced, the pressure in the kettle was kept at 0.6MPa, the temperature was kept at about 40°C, and the reaction was carried out for 4h. Filter, wash the filter cake with 100ml of ammonia-ammonium chloride buffer solution, separate the filtrate, wash the water phase with 300ml of ethyl acetate, cool the water phase to below 10°C with an ice-water bath, adjust to pH 5 with 2N hydrochloric acid, and gradually precipitate solids , stirred at below 10°C for 2h, fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com