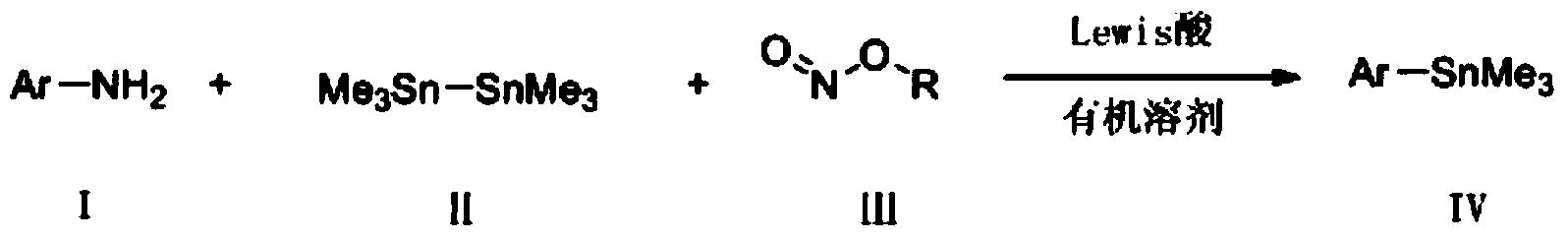Preparation method for aryl tin compound
A technology of tin compounds and compounds, applied in the direction of tin organic compounds, etc., can solve problems such as harsh reaction conditions, unfriendly environment, complicated operation, etc., and achieve the effects of low preparation cost, low reaction cost and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
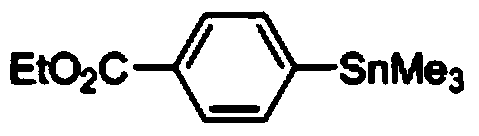
[0037] Synthesis of ethyl p-trimethylstannyl benzoate
[0038] Add 50mg (ie 0.3mmol) ethyl p-aminobenzoate, 62mg (ie 0.36mmol) p-toluenesulfonic acid, 1.5mL 1,2-dichloroethane into a 10mL long tube reaction bottle, and then weigh 62mg (ie 0.6mmol) tert-butyl nitrite and 108mg (ie 0.33mmol) hexamethyldistannane, react at 0°C for 4h, concentrate after reaction, wash with petroleum ether: ethyl acetate = 50:1 Purification by deagent column chromatography can obtain ethyl p-trimethylstannyl benzoate, and its structure is shown in the following formula:
[0039]
[0040] The compound is a light yellow liquid with a yield of 76%, and its NMR data are as follows:
[0041] 1 HNMR (400MHz, CDCl 3 )δ7.98(d,J=7.2Hz,2H),7.57(d,J=7.2Hz,2H),4.37(q,J=6.7Hz,2H),1.39(t,J=6.7Hz,3H) ,0.31(s,9H); 13 CNMR (100MHz, CDCl 3 )δ166.9, 149.4, 135.7, 130.2, 128.5, 60.9, 14.4, -9.5;
Embodiment 2
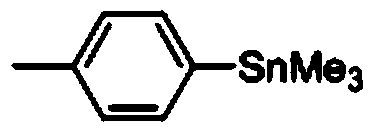
[0043] Synthesis of p-methylphenyltrimethylstannane
[0044] Add 32mg (ie 0.3mmol) p-toluidine, 62mg (ie 0.36mmol) p-toluenesulfonic acid, 1.5mL 1,2-dichloroethane into a 10mL long tube reaction bottle, and then weigh 62mg (ie 0.6mmol) of tert-butyl nitrite and 108mg (ie 0.33mmol) of hexamethyldistannane were reacted at 30°C for 4h, concentrated after the reaction, and purified by column chromatography using petroleum ether as eluent to obtain p-formazan phenyl trimethylstannane, its structure is shown in the following formula:
[0045]
[0046] The compound is a colorless liquid with a yield of 53%, and its NMR data are as follows:
[0047] 1 HNMR (400MHz, CDCl 3 )δ7.39(d,J=7.5Hz,2H),7.17(d,J=7.5Hz,2H),2.34(s,3H),0.27(s,9H); 13 CNMR (100MHz, CDCl 3 )δ138.2, 138.0, 135.8, 128.9, 21.4, -9.5;
Embodiment 3
[0049] Synthesis of p-Methoxyphenyltrimethylstannane
[0050] Add 37mg (ie 0.3mmol) p-methoxyaniline, 62mg (ie 0.36mmol) p-toluenesulfonic acid, 1.5mL 1,2-dichloroethane into a 10mL long tube reaction bottle, and then weigh 62mg (ie 0.6mmol) tert-butyl nitrite and 108mg (ie 0.33mmol) hexamethyldistannane, reacted at 25°C for 3h, concentrated after the reaction, and purified by column chromatography with petroleum ether as eluent to obtain P-methoxyphenyltrimethylstannane, its structure is shown in the following formula:
[0051]
[0052] The compound is a colorless liquid with a yield of 49%, and its NMR data are as follows:
[0053] 1 HNMR (400MHz, CDCl 3 )δ7.41(d,J=8.4Hz,2H),6.92(d,J=8.4Hz,2H),3.80(s,3H),0.26(s,9H); 13 CNMR (100MHz, CDCl 3 )δ159.9, 136.9, 132.4, 114.0, 55.0, -9.5;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com