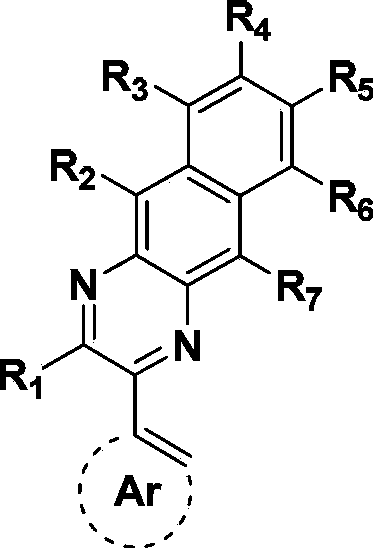
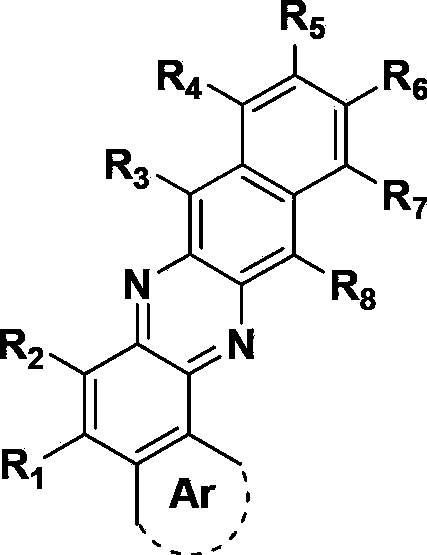
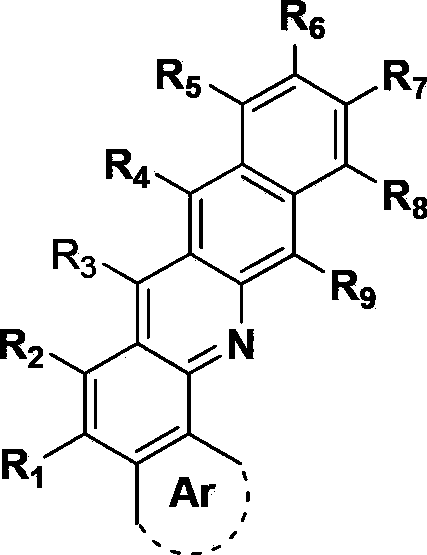
Metal complex and application thereof in organic electroluminescent device
A technology of complexes and metal iridium, applied in the direction of electric solid devices, electrical components, luminescent materials, etc., can solve the problems of efficiency roll-off, etc., and achieve the effects of short luminous life, high luminous efficiency, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Compound C1-1
[0056] Reaction formula:
[0057]
[0058] process:
[0059] IrCl 3 ·xH 2 O (58%Ir) and 2.2 times the chemical equivalent of the ligand were dissolved in a mixed solvent of ethylene glycol methyl ether and deionized water (v / v=3 / 1). Stir under reflux at 100° C. for 24 hours under an Ar atmosphere. Cool to room temperature and filter, wash the filter cake with deionized water until neutral, then rinse the filter cake with 10mL ethanol and 200mL diethyl ether in sequence. Finally, the filter cake was dissolved with dichloromethane, the filtrate was collected, the solvent was removed by rotary evaporation, and vacuum-dried at 70°C for 5 hours to obtain an ocher solid with a yield of 89%, which was directly put into the next reaction without further purification. 1 H-NMR (CDCl 3 ,300MHz,δ[ppm]):2.81(s,3H),5.66~5.69(d,1H),6.13~6.18(t,1H),6.74~6.79(t,1H),7.51~7.61(m,2H ),7.74~7.78(t,1H),7.95~7.98(d,1H),8.06(s,1H),8.86(s,1H).ESI-MS[m / z]:1...
Embodiment 2
[0063] Example 2: Compound C1-2
[0064]
[0065] The acetylacetone in Example 1 was replaced by picolinic acid, and the complex was obtained by a similar method with a yield of 45%.
[0066] 1 H-NMR (CDCl 3 ,600MHz,δ[ppm]):2.68(s,6H),7.41(t,2H),7.51(m,4H),7.65(m,4H),7.79(d,2H),7.88(t,1H) ,7.98(t,1H),8.06(s,4H),8.16(d,4H),8.29(d,1H),9.01(d,1H).
[0067] ESI-MS[m / z]:854[M+H] + .Elemental analysis (C44H30N5IrO2): Anal. Calcd.: C, 61.96; H, 3.55; N, 8.21. Found: C, 62.02; H, 3.60; N, 8.19.
Embodiment 3
[0068] Example 3: Compound C1-3
[0069]
[0070]0.76g (0.5mmol) of dichloro-bridged intermediate, 0.30g (1.1mmol) of the main ligand and 0.28g (1.1mmol) of silver trifluoromethanesulfonate were dissolved in 50mL of ethylene glycol monomethyl ether. The reaction system was protected by argon, shielded from light, and stirred at reflux for 24 hours. After cooling to room temperature, filter and rinse with a small amount of ethylene glycol monomethyl ether. Then elute with dichloromethane, collect the filtrate, distill off the solvent, separate by column chromatography, and collect the black product band. After concentration, it was recrystallized with dichloromethane / ether to obtain a black solid with a yield of 30%.
[0071] 1 H-NMR (CDCl 3 ,300MHz,δ[ppm]):2.81(s,3H),5.66~5.69(d,1H),6.13~6.18(t,1H),6.74~6.79(t,1H),7.51~7.61(m,2H ),7.74~7.78(t,1H),7.95~7.98(d,1H),8.06(s,1H),8.86(s,1H).
[0072] ESI-MS[m / z]:1001[M+H] + .Elemental analysis (C57H39N6Ir): Anal. Calcd.: C,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com