Orthophosphate scintillator material with beta-K2SO4 (potassium sulfate) structure and preparation method and application thereof
A scintillator material, -K2SO4 technology, applied in analytical materials, luminescent materials, fluorescence/phosphorescence, etc., can solve the problems of low luminous efficiency, short afterglow, easy deliquescence, etc., and achieve short luminous life, stable structure and performance, cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
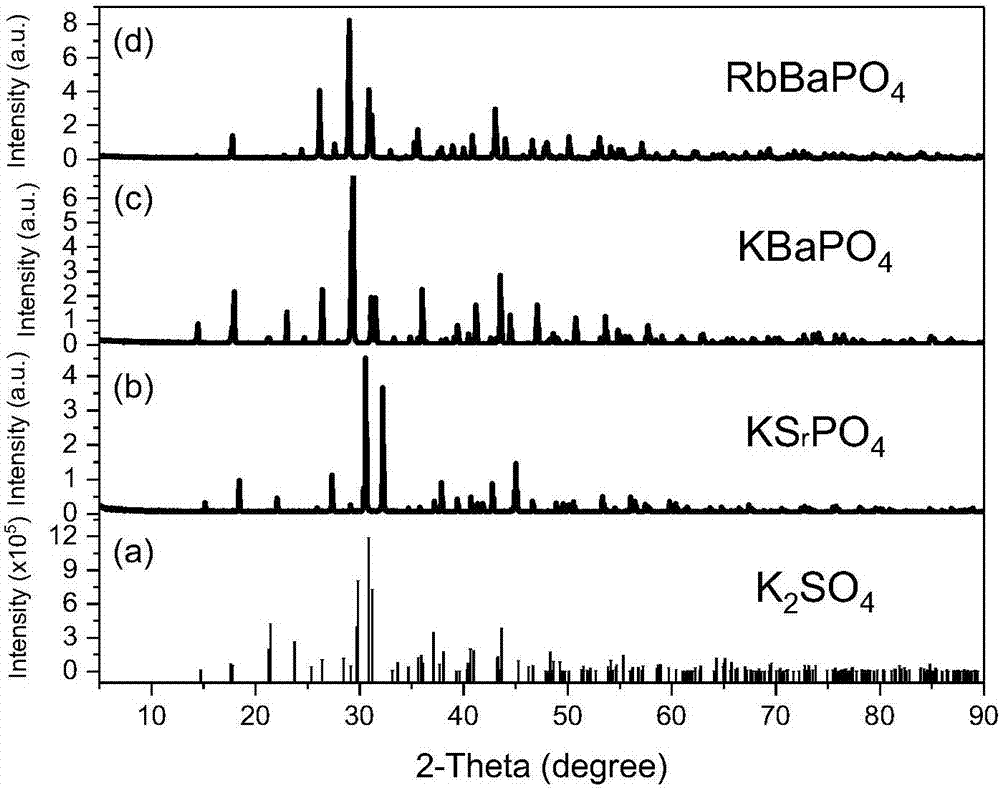
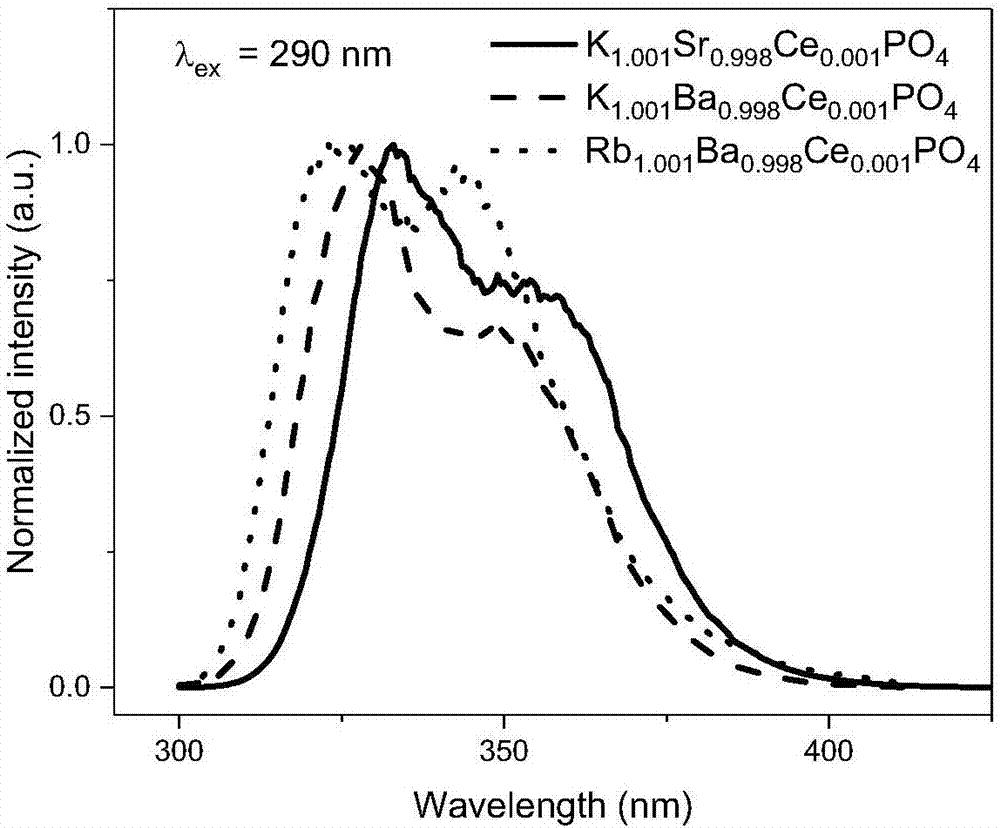
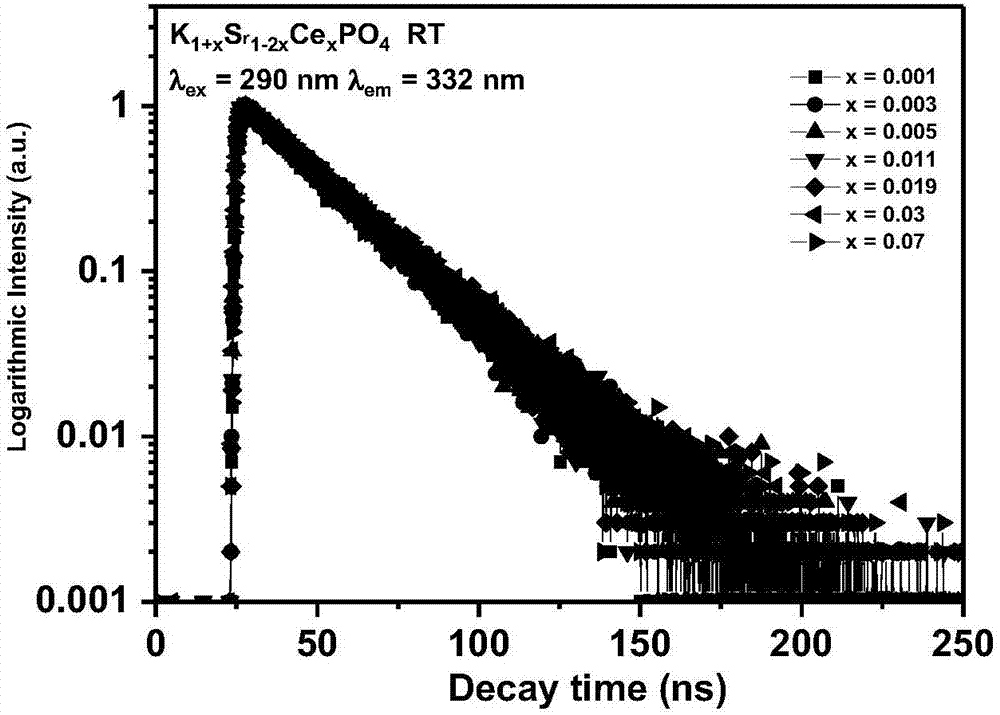
[0043] Weigh potassium dihydrogen phosphate (KH 2 PO 4 ) 0.6905 g, strontium carbonate (SrCO 3 ) 0.7367 g, potassium carbonate (K 2 CO 3 ) 0.003g and ceria (CeO 2 ) 0.0009 g in an agate mortar and grind for 20 minutes to mix evenly, then put it into a corundum crucible, put the corundum crucible into a large corundum crucible with a carbon block, and roast the reaction at a high temperature in a CO reducing atmosphere. The specific heating procedure is as follows : Take 5 hours to raise the temperature from room temperature to 1300°C, and keep it warm for 3 hours; wait for the sample to cool naturally to room temperature to obtain the final product, and its chemical composition expression is K 1.001 Sr 0.998 Ce 0.001 PO 4 .
Embodiment 2
[0045] Weigh potassium dihydrogen phosphate (KH 2 PO 4 ) 0.6905 g, barium carbonate (BaCO 3 ) 0.9847 g, potassium carbonate (K 2 CO 3 ) 0.003g and ceria (CeO 2 ) 0.0009 g in an agate mortar and grind for 20 minutes to mix evenly, then put it into a corundum crucible, put the corundum crucible into a large corundum crucible with a carbon block, and roast the reaction at a high temperature in a CO reducing atmosphere. The specific heating procedure is as follows : Take 5 hours to raise the temperature from room temperature to 1300°C, and keep it warm for 3 hours; wait for the sample to cool naturally to room temperature to obtain the final product, and its chemical composition expression is K 1.001 Ba 0.998 Ce 0.001 PO 4 .
Embodiment 3
[0047] Weigh diammonium hydrogen phosphate ((NH 3 ) 2 HPO 4 ) 0.5582 g, barium carbonate (BaCO 3 ) 0.7878 g, rubidium carbonate (Rb 2 CO 3 ) 0.4904 g and ceria (CeO 2 ) 0.007 g in an agate mortar and ground for 20 min to mix evenly, then put it into a corundum crucible, put the corundum crucible into a large corundum crucible with carbon blocks, and roast the reaction at high temperature in a CO reducing atmosphere. The specific heating procedure is as follows : It takes 4 hours to raise the temperature from room temperature to 1000°C, and keep it warm for 6 hours; after the sample is naturally cooled to room temperature, the final product is obtained, and its chemical composition expression is Rb 1.001 Ba 0.998 Ce 0.001 PO 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com