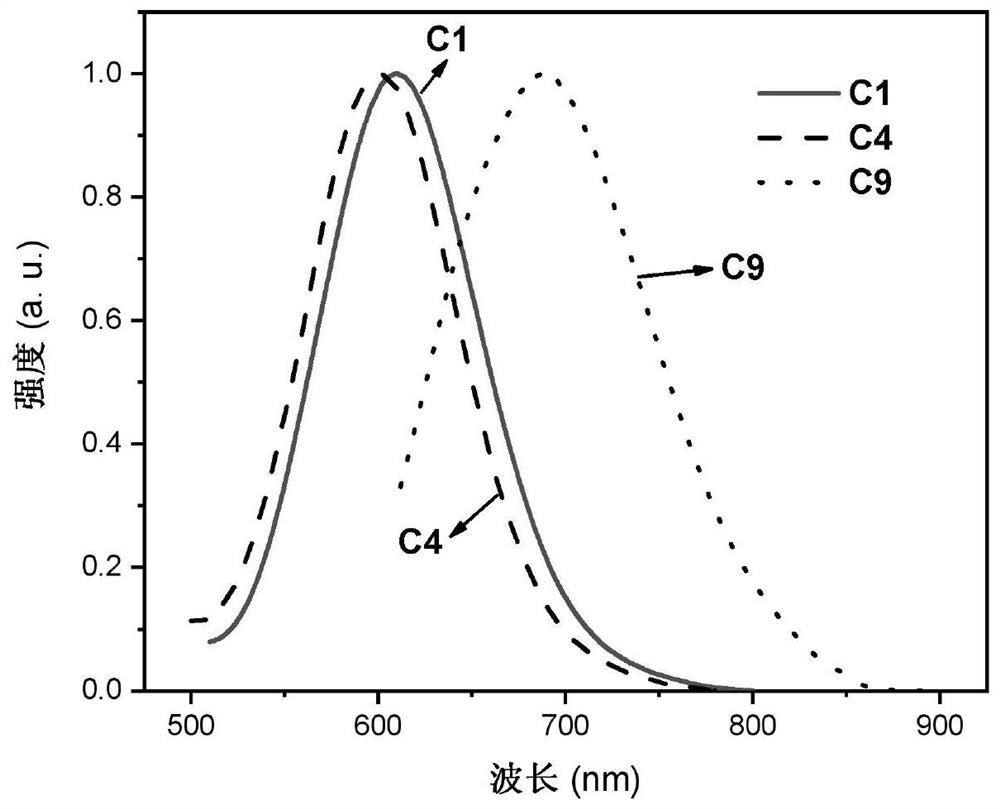
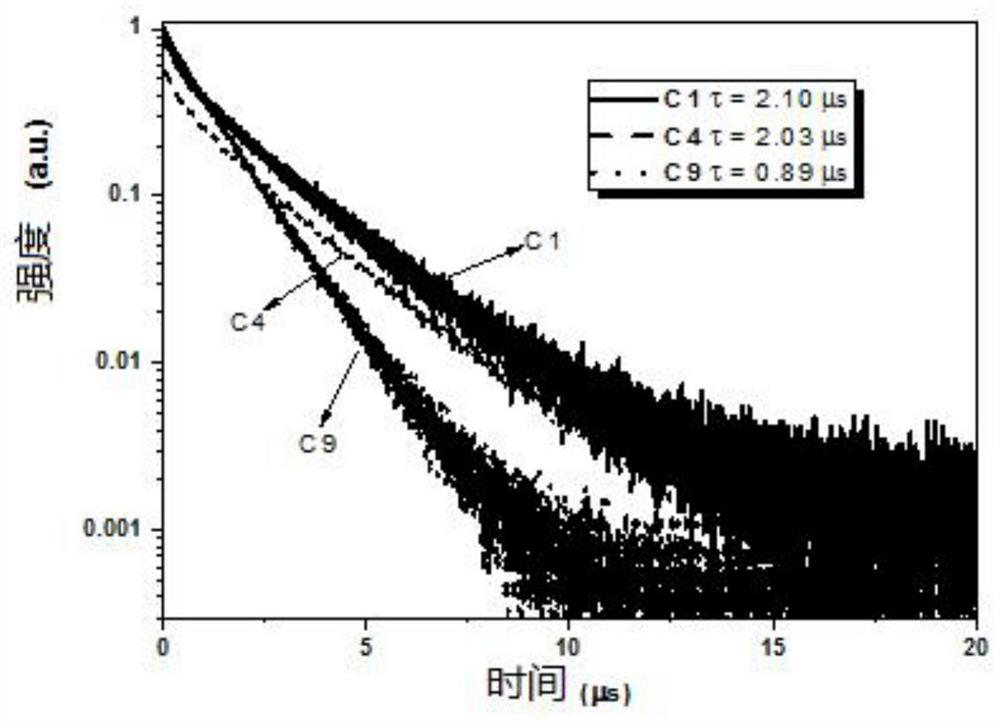
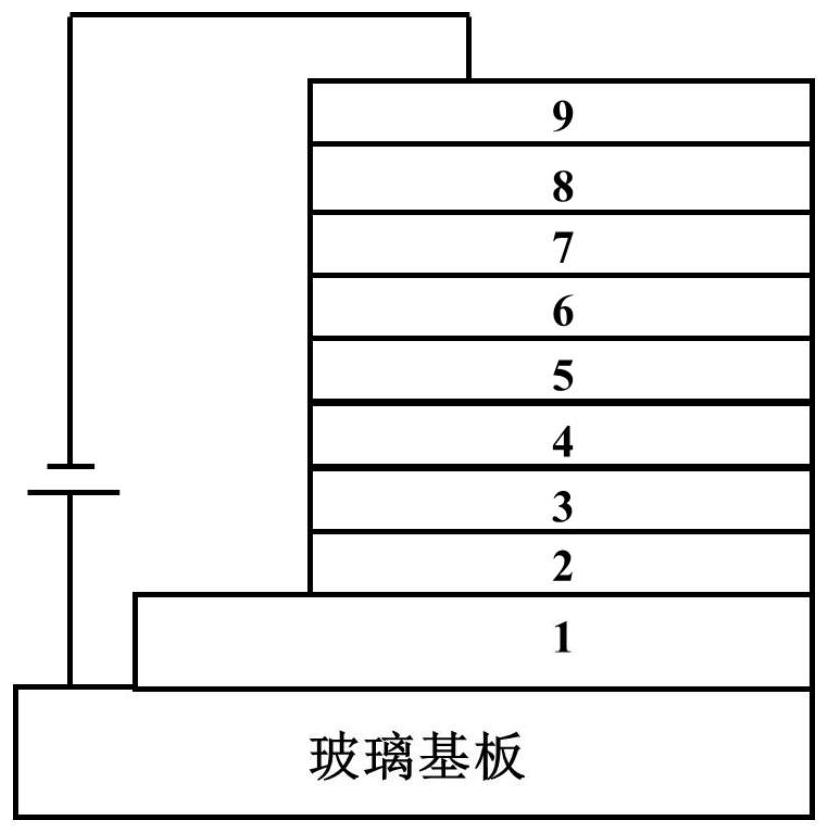
Binuclear metal platinum complex and organic electroluminescent device
A platinum complex and binuclear metal technology, applied in the field of binuclear metal platinum complexes and organic electroluminescence devices, can solve the problems of device stability under unfavorable electric field, unfavorable device service life, limited luminescence brightness, etc., and achieves suppression of non-radiation Transition, short luminous lifetime, and small roll-off of device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] This embodiment provides the preparation method of compound C1, and its synthetic route is as follows:
[0091]
[0092] The preparation method of the compound C1 comprises the following steps:
[0093] 1) Preparation of Intermediate I-2
[0094] Put potassium tetrachloroplatinate (3.6g, 8.7mmol) and a magnetic stirrer in a two-necked flask, and evacuate argon three times in a circular vacuum, and inject 45mL of degassed ethylene glycol ether and 15mL of water into the reaction system , injected compound Ⅰ-1 (2.4g, 15mmol), heated to 120 degrees Celsius, reacted for 24 hours, cooled to room temperature, distilled off the solvent under reduced pressure, cooled to room temperature, added 25 mL of ethanol, collected the solid by filtration, and dried in vacuum for 24 hours to obtain Yellow solid I-2 (68% yield).
[0095] 2) Preparation of compound C1
[0096] Mix Intermediate I-2 (783.1 mg, 1.0 mmol), α-carboline (420.2 mg, 2.5 mmol), and anhydrous potassium carbonat...
Embodiment 2
[0098] This embodiment provides the preparation method of compound C4, and its synthetic route is as follows:
[0099]
[0100] The preparation method of the compound C4 comprises the steps of:
[0101] 1) The preparation method of intermediate II-2 is the same as that of intermediate I-2, except that compound I-1 is replaced by II-1, and intermediate II-2 obtained is a dark green solid (yield 70%).
[0102] 2) The preparation method of compound C4 is the same as that of compound C1, the difference is that the compound I-2 is replaced by II-2, and the obtained compound C4 is a red solid (yield 30%). 1 H NMR (500MHz, deuterated chloroform) δ (ppm) 8.55 (d, J = 5.8Hz, 2H), 8.29 (d, J = 8.1Hz, 2H), 8.27–8.21 (m, 2H), 8.04 (d, J=7.7Hz, 2H), 7.82(d, J=5.7Hz, 2H), 7.68(d, J=8.4Hz, 2H), 7.48(q, J=8.9, 8.2Hz, 4H), 7.20(t, J = 7.5Hz, 2H), 6.84–6.76 (m, 2H), 6.34 (t, J = 6.6Hz, 2H), 6.30–6.20 (m, 2H), 5.50 (d, J = 8.6Hz, 2H). Mass spectrum: [M+H]: 1105.1484.
Embodiment 3
[0104] This embodiment provides the preparation method of compound C9, and its synthetic route is as follows:
[0105]
[0106] The preparation method of the compound C9 comprises the steps of:
[0107] 1) The preparation method of intermediate III-2 is the same as that of intermediate I-2, except that compound I-1 is replaced by III-1, and intermediate III-2 obtained is a dark green solid (yield 70%).
[0108] 2) The preparation method of compound C9 is the same as that of compound C1, except that compound I-2 is replaced by III-2, and compound C9 is obtained as a magenta solid (yield 40%). 1 H NMR (400MHz, deuterated chloroform) δ (ppm) 8.66(s, 2H), 8.32(d, J=8.1Hz, 2H), 8.26(d, J=7.5Hz, 2H), 8.06(d, J= 7.7Hz, 2H), 7.98(d, J=8.7Hz, 2H), 7.72(d, J=6.1Hz, 2H), 7.60(q, J=8.6, 8.1Hz, 4H), 7.45(dt, J= 15.0,7.2Hz,4H),7.18(t,J=7.4Hz,2H),7.16–7.08(m,2H),6.81(t,J=6.4Hz,2H),6.67(d,J=5.9Hz, 2H), 6.16 (t, J=8.7Hz, 2H), 5.85 (d, J=9.4Hz, 2H). Mass spectrum: [M+H]: 1169.1980.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com