A Novel Thermally Activated Delayed Fluorescent Material Based on Excimer Luminescence and Its Application
A thermal activation delay and luminescent material technology, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of long luminous life of DF, roll-off efficiency of organic light-emitting diodes, and difficulties in the composition of exciplex-type TADF materials. Control and other issues to achieve the effect of reducing ΔEST
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
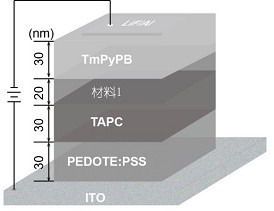
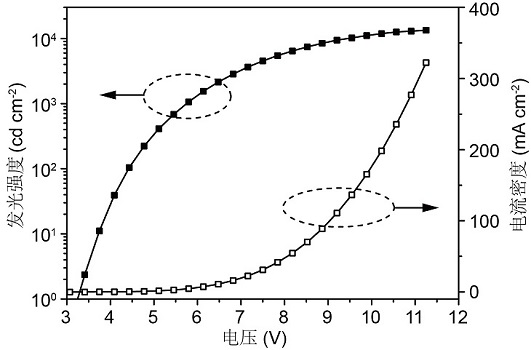
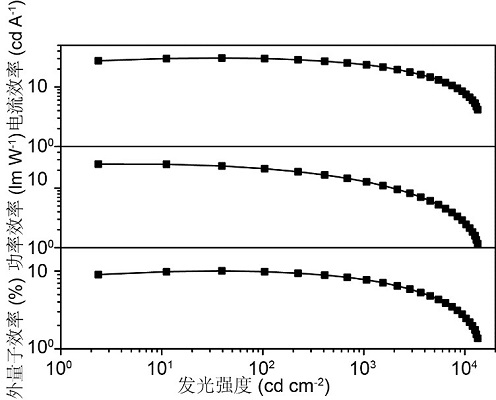
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of 3,5-dicyanobenzoyl chloride:
[0031]
[0032] First, add 3,5-dibromobenzoic acid (5.6 g, 20.0 mmol) into a 100 ml round-bottomed two-necked flask equipped with a magnet, then add 15 ml of methanol, and slowly add thionyl chloride (3.0 ml, 40.0 mmol) and stirred at 70°C for 6 hours under the protection of argon. After the reaction, the reaction mixture was concentrated to obtain DBPOMe (6 g) with a yield of 100%.
[0033] Put the above product (3 g, 10.0 mmol) and cuprous cyanide (2.26 g, 25.0 mmol) into a 100 ml round-bottomed two-necked flask with a magnet, and add 40 ml of ultra-dry N,N-dimethylformaldehyde amide (DMF), and stirred at 180°C for 24 hours under the protection of argon. After the reaction was cooled to room temperature, the reaction solution was poured into a 500 ml separatory funnel, washed with saturated brine (200 ml) and extracted with dichloromethane. The collected organic phase was treated with anhydrous magn...
Embodiment 2
[0036]Example 2: Preparation of Compound 1 (5-(9H-carbazole-9-carbonyl)isophthalonitrile)
[0037]
[0038] Under the condition of 0ºC, carbazole (0.84 g, 5 mmol) and 60% NaH (0.24 g, 6 mmol) were dissolved in anhydrous THF (20 mL) and stirred at room temperature for 30 minutes, then 3, 5-Dicyanobenzoyl chloride (1.1 ml, 5 mmol). After stirring for 10 minutes, return to room temperature and stir for another 2 hours. The product was treated with CH 2 Cl 2 Extract three times and collect the organic phase, the organic phase with anhydrous magnesium sulfate (MgSO 4 )dry. The solvent was removed by distillation under reduced pressure, and the obtained crude product was purified by column chromatography, and the eluent was petroleum ether and dichloromethane in a volume ratio of 3:1. The product was a yellow-green powder, which was recrystallized several times from dichloromethane / hexane to give yellow-green crystals (1.1 g, yield 70%).
Embodiment 3
[0039] Embodiment 3: Preparation of compound 2
[0040]
[0041] At 0ºC, acridine (1.05 g, 5 mmol) and 60% NaH (0.24 g, 6 mmol) were dissolved in anhydrous THF (20 mL) and stirred at room temperature for 30 minutes, then 3, 5-Dicyanobenzoyl chloride (1.1 ml, 5 mmol). After stirring for 10 minutes, return to room temperature and stir for another 2 hours. The product was treated with CH 2 Cl 2 Extract three times and collect the organic phase, the organic phase with anhydrous magnesium sulfate (MgSO 4 )dry. The solvent was removed by distillation under reduced pressure, and the obtained crude product was purified by column chromatography, and the eluent was petroleum ether and dichloromethane in a volume ratio of 3:1. The product was a yellow-green powder, which was recrystallized several times from dichloromethane / hexane to give yellow-green crystals (1.1 g, yield 65%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com