Styrene-acrylic sucrose ester glycoside compounds of prunus tomentosa, and preparation method and application thereof
A technology of phenylpropose and compound, applied in the field of medicine, can solve the problems of urgency, strong side effects and drug resistance of tumor drugs, and achieve the effects of good anti-tumor activity, good anti-tumor activity and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
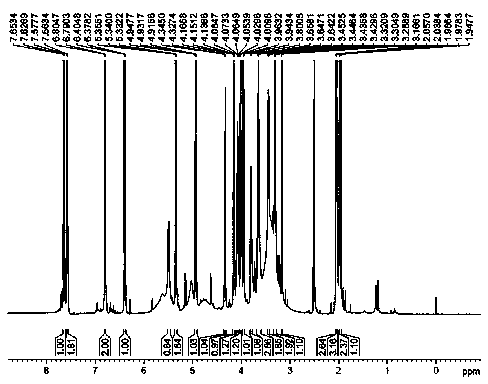
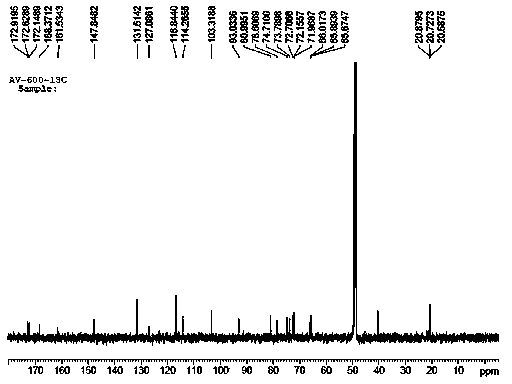
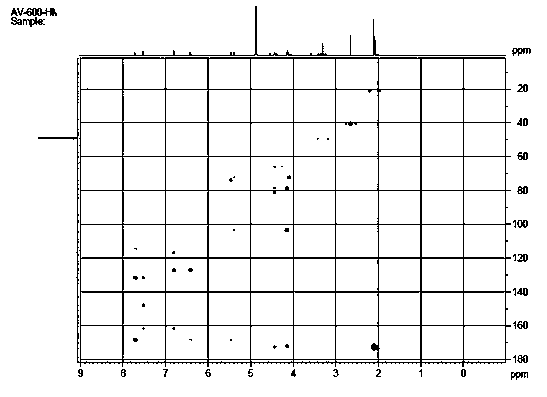
[0047]Take 9.5kg of dry cherry leaves and reflux them with 60L of 70% ethanol to extract three times, each time for two hours, combine the extracts and concentrate to obtain 1.2kg of extract, suspend the extract with 3L of water, and use petroleum ether: suspension =1:1 and ethyl acetate:suspension=1:1 extraction. Concentrate the ethyl acetate extract to obtain 120 g of extract. The extract adopts fast decompression column chromatography, and is gradually eluted with dichloromethane-methanol system 50:1, 30:1, 15:1, 10:1, 5:1, 2:1, 1:1, and every 500ml is One fraction, a total of 21 fractions were collected, detected by silica gel thin layer chromatography, and combined into 4 parts A1~A4 (50:1 eluent was A1, 30:1~10:1 was combined into A2, 8:1~ 2:1 combined into A3, 1:1 eluent is A4), in which A2 is the main fraction with a weight of 75g, and A2 is purified by CHP20 column chromatography with 90% methanol-water (or ethanol-water) to remove part of the pigment to obtain B1 (...
preparation Embodiment 2
[0050] Take 9 kg of dry cherry leaves and extract them three times with 60L of 65% ethanol under reflux, for three hours each time, combine the extracts and concentrate to obtain 1.3 kg of extract, suspend the extract with 3.5L of water, and use petroleum ether: suspension =1:1 and ethyl acetate:suspension=1:1 extraction. Concentrate the ethyl acetate extract to obtain 130 g of extract. The extract adopts fast decompression column chromatography, and is gradually eluted with dichloromethane-methanol system 50:1, 30:1, 15:1, 10:1, 5:1, 2:1, 1:1, and every 500ml is One fraction, a total of 21 fractions were collected, detected by silica gel thin layer chromatography, and combined into 4 parts A1~A4 (50:1 eluent was A1, 30:1~10:1 was combined into A2, 8:1~ 2:1 merged into A3, 1:1 eluent is A4), in which A2 is the main fraction with a weight of 84g, and A2 is passed through CHP20 column chromatography to remove part of the pigment with 90% methanol-water (or ethanol-water) to obt...
preparation Embodiment 3
[0053] Take 8.5kg of dry cherry leaves and extract them three times with 55L of 70% ethanol under reflux for two hours each time, combine the extracts and concentrate to obtain 1.1kg of extract, suspend the extract with 3.0L of water, and then use petroleum ether: suspend Solution=1:1 and ethyl acetate:suspension=1:1 extraction Concentrate the ethyl acetate extract to obtain extract 110g. The extract adopts rapid decompression column chromatography, and gradually elutes with dichloromethane-methanol system 50:1, 30:1, 15:1, 10:1, 5:1, 1:1, and every 500ml is a fraction, a total of 18 fractions were collected, detected by silica gel thin layer chromatography, and combined into 4 parts A1~A4 (50:1 eluent was A1, 30:1~10:1 was combined into A2, 8:1~2:1 was combined A3, 1:1 eluent is A4), wherein A2 is the main fraction with a weight of 79g, and A2 is obtained by removing part of the pigment by CHP20 column chromatography with 90% methanol-water (or ethanol-water) to obtain B1 (71...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com