Method for efficiently acquiring inductive pluripotent stem cells (iPSCs)
A pluripotent stem cell, inducible technology, applied in the field of cells, can solve the problems of low efficiency and long cycle of pig iPSCs, and achieve the effect of improving efficiency, shortening cycle and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
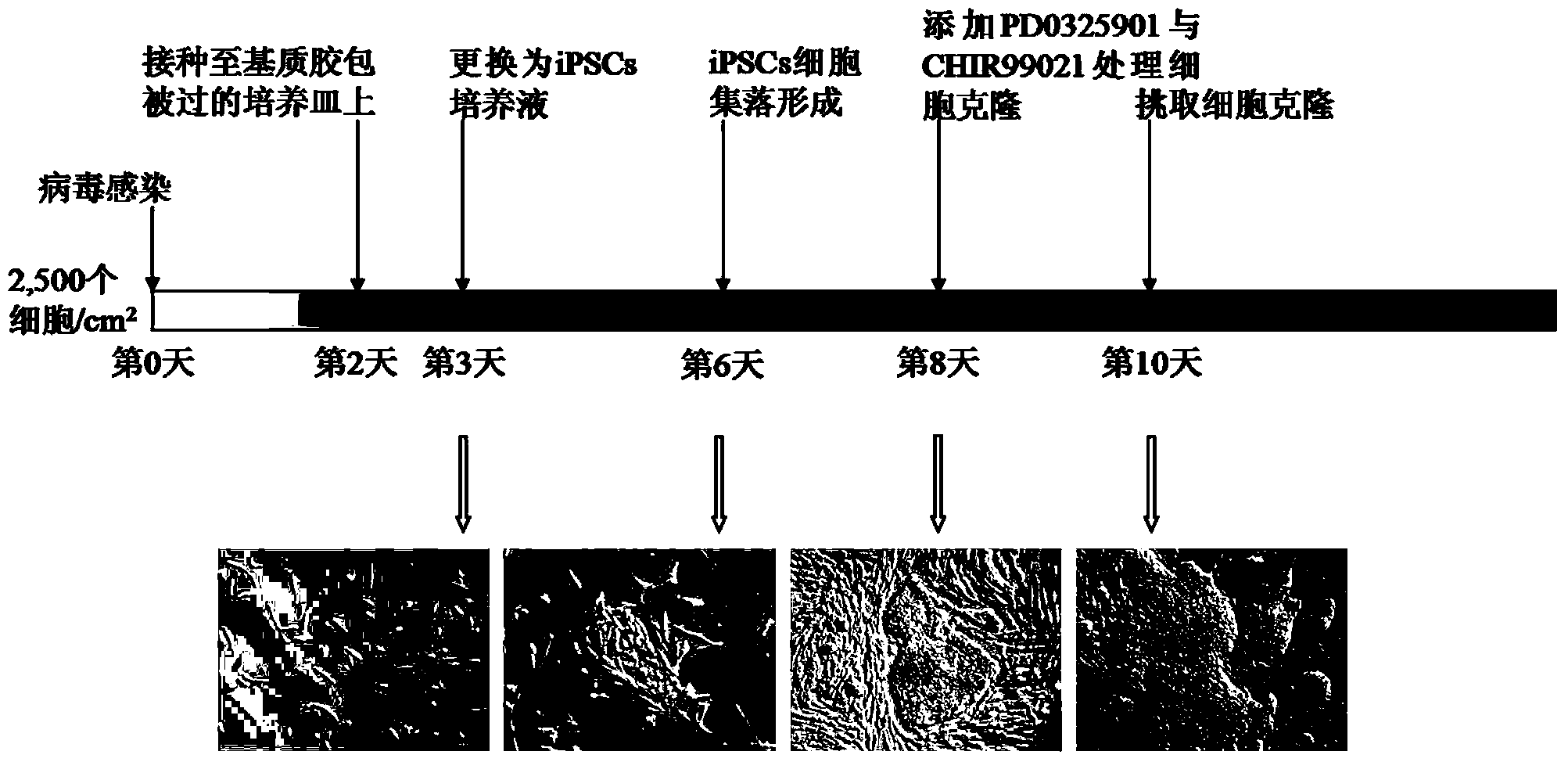
[0046] Example 1. Preparation and cultivation of cells
[0047] 1.1 Culture of porcine adipose stem cells (ADSCs)
[0048]Separate the adipose tissue from the pig's back and abdomen subcutaneously, remove blood vessels and muscle residues, wash thoroughly with DPBS buffer (Gibco), then use surgical scissors to fully cut the adipose tissue until there is almost no shear resistance, and transfer to the centrifuge. In the tube, add 0.09% type I collagenase digestion solution (Sigma company) equivalent to 2 to 3 times the total volume of adipose tissue, and place it in a water bath at 37°C for shaking and digestion; after the tissue suspension is gelatinized, use Pasteur The pipette was blown repeatedly to disperse tissue debris, centrifuged at room temperature at 1,200rpm for 5min, discarded the mature adipose tissue in the upper layer of the centrifuge tube and the digestive juice in the middle layer, and then used DMEM / F-12 basal medium containing 10% fetal bovine serum (HyClon...
Embodiment 2
[0053] Example 2. Infection of porcine ADSCs with viral vectors
[0054] According to the method described in Example 1, low-passage porcine ADSCs were inoculated in cell culture flasks at 37°C, 5% CO 2 When the confluence reached 80-90% under conventional culture conditions, digested with 0.25% trypsin-EDTA (Gibco) at 37°C to form a single-cell suspension, and infected 1×10 cells with the collected virus supernatant. 5 ADSCs, the multiplicity of infection (MOI) was 3 (the titer of the virus used was 5-10×10 6 IU / mL, the four viruses carrying the pluripotency factor cDNA were mixed according to 1:1:1:1), and then inoculated into 6-well culture plates, and continued to store at 37°C, 5% CO 2 cultured under conventional culture conditions. The viral supernatant was transfected into 293T cells (Fugene HD by a conventional method) with a drug-inducible (RevTet-On) lentiviral expression vector (SiDanSai Company) containing cDNA of human Oct4, Sox2, Klf4 and c-Myc. , Roche Compan...
Embodiment 3
[0055] Example 3. Continued culture and clone screening of infected cells
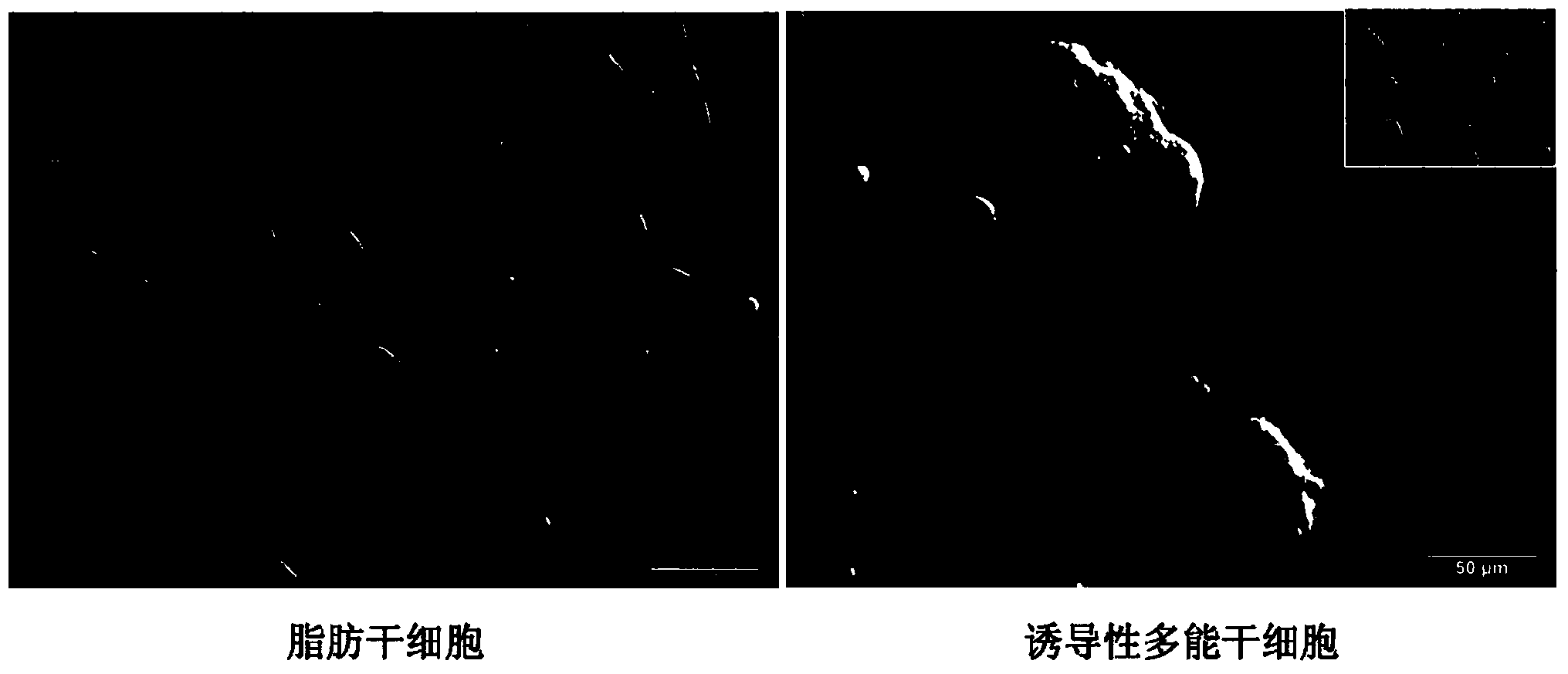
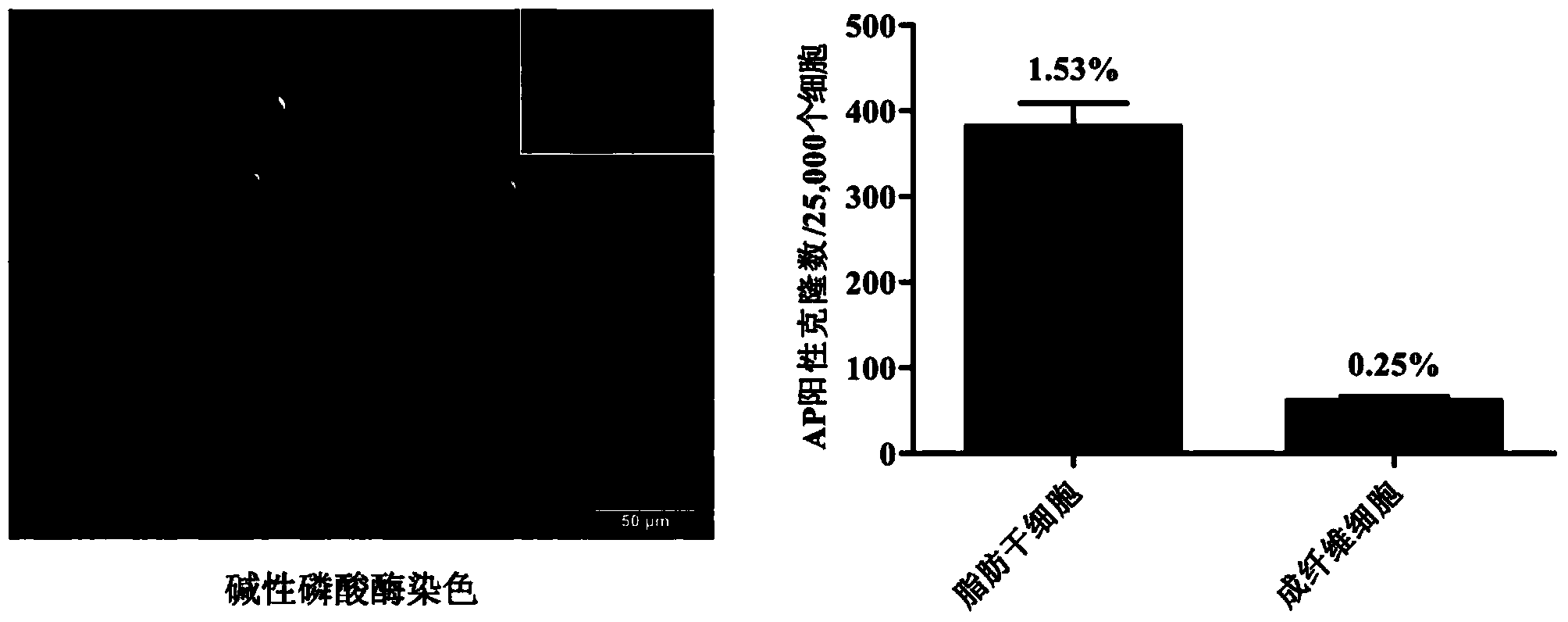
[0056] On the second day after infection, the culture wells were washed twice with DPBS buffer, and then the infected ADSCs were digested into a single-cell suspension with 0.25% trypsin-EDTA (Gibco Company) at 37°C, and the cells were divided into 2,500 cells. / cm 2 The density was inoculated into a new 6-well culture plate, and a total of 4 culture wells were inoculated. The surface of the culture plate was pre-coated with Matrigel (BD Pharmingen Company), and the ADSCs complete medium was continued to be used. Among the parallel samples of 4 wells here, 1 well was used for alkaline phosphatase (AP) staining (SiDanSai Company), and the number of positive clones was counted, and the other 3 wells were used for clone selection. The experiment was repeated three times. On the third day after infection, the ADSCs complete medium was replaced with the porcine iPSCs serum-free complete medium described in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com