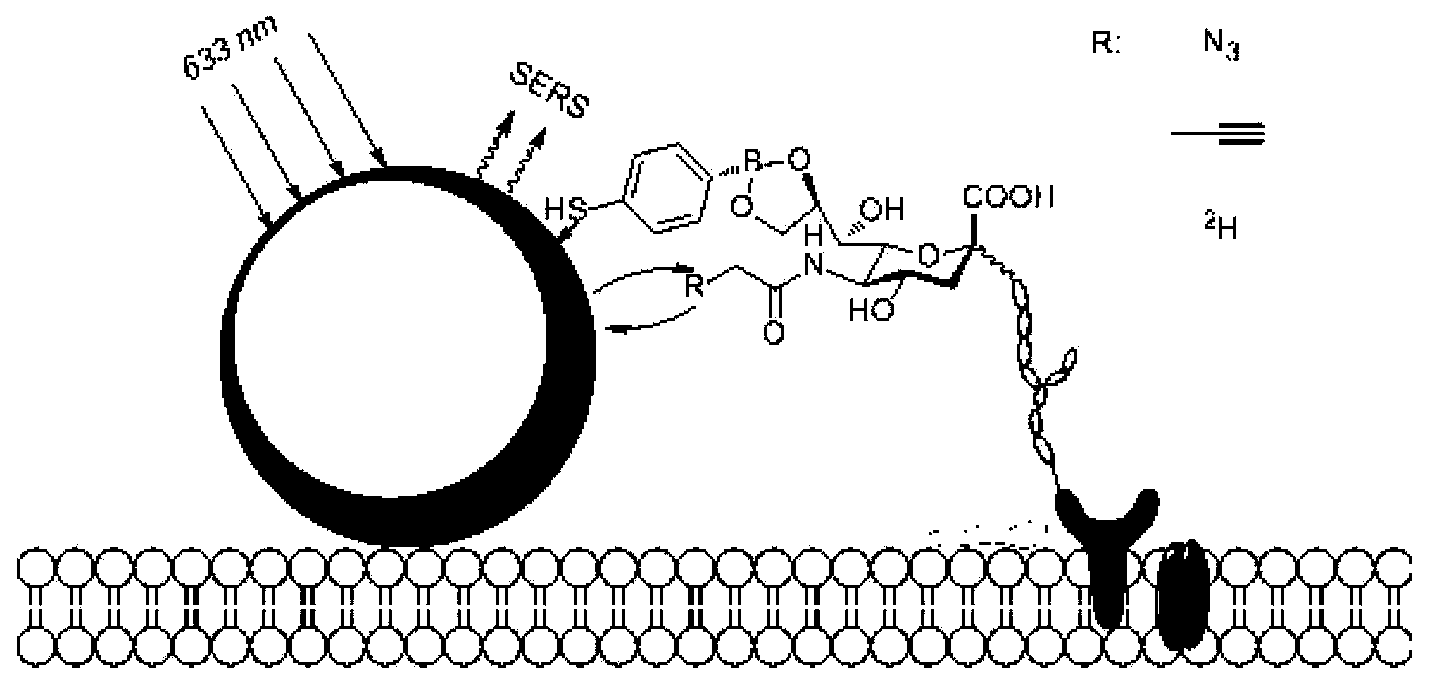
4-mercaptophenyl boronic acid-modified gold nanoparticles and method for detecting sugar marker on cell surface by using gold nanoparticles
A technology of gold nanoparticles, mercaptophenylboronic acid, applied in the field of chemical glycobiology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0032] The synthesis of embodiment 1AuMPBA
[0033] Gold nanoparticles of 120 nm were synthesized using a two-step method of sodium citrate reduction. Step 1, 100ml 0.01% HAuCl 4 The aqueous solution was heated to reflux, and 1ml of 1% sodium citrate solution was quickly added under rapid stirring. After reflux for 15 minutes, cool at room temperature to obtain 40nm AuNPs seed crystals. In the second step, 4ml of 40nm AuNPs seed crystals were added to 56ml of ultrapure water, and 0.9ml of 1% sodium citrate and 0.9ml of 1% HAuCl were added under stirring. 4 and 1.4ml of 10mM hydroxylamine hydrochloride solution, stirred at room temperature for 1h to obtain 120nm AuNPs. Finally, MPBA was used to replace citrate in ethanol solution to form 120nm AuMPBA. The specific method is to centrifuge 120 nm AuNPs at 3000 rpm for 10 min, discard the supernatant, add 2 mM MPBA ethanol solution, and stir at room temperature overnight (eg, 8-24 hours). Before use, AuMPBA was centrifuged at...
Embodiment 2
[0034] Example 2 Detection of Raman labels (azide and alkyne) in cell lines (HeLa and CHO)
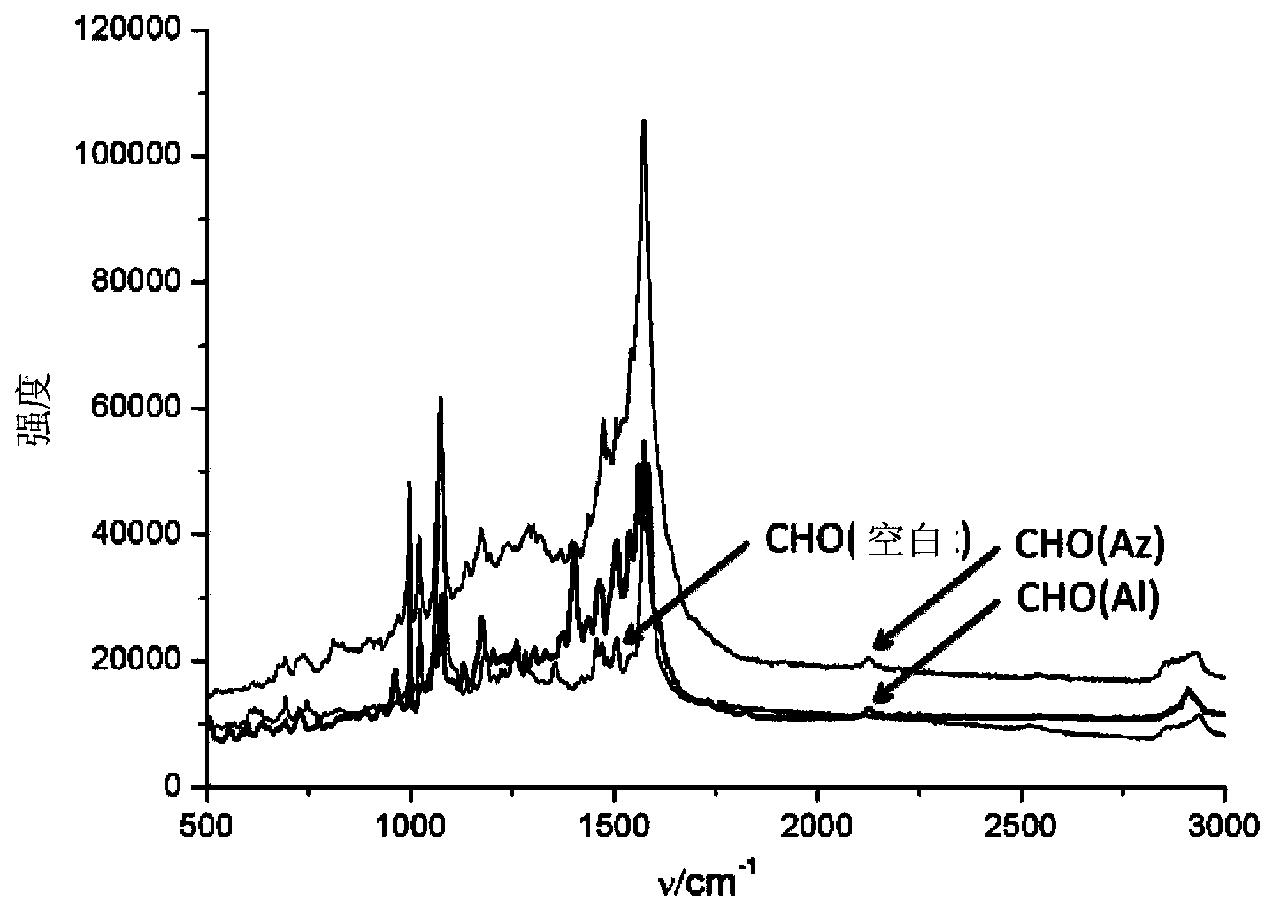
[0035] Modification of AuMPBA to the cell surface: add 100 μM Ac 4 ManNAz or Ac 4 ManNAl’s DMEM medium cultured HeLa or CHO cells in an incubator at 37 degrees Celsius for three days, washed three times with phosphate buffer solution (PBS, pH=7.4) to wash off unnatural sugars that did not enter the cells, and then washed with AuMPBA’s PBS solution at 37 degrees Celsius Incubate the cells for half an hour to bind the gold nanoparticles to the surface of the cell membrane, wash three times with phosphate buffer solution (PBS, pH=7.4) and seal the slide, and finally use a Raman spectrometer to detect the signal of the Raman label on the surface of the cell membrane, using a 50% intensity 633nm laser The signal is collected for 10s. For the results, please refer to figure 2 , image 3 and Figure 4. in, figure 2 is the dark field imaging of the Raman spectrometer, the bright spot ...
Embodiment 3
[0039] Example 3 Detection of Raman label (deuterated methyl group) in cell lines (HeLa and CHO)
[0040] Although the detection of the Raman labels in Example 2 can prove that they themselves are dynamically expressed on the cell membrane, because their physical and chemical properties have changed, they may enter the cell membrane through a different metabolic pathway from natural sugars and cannot represent natural sugars. Dynamic expression. Deuterated compounds have the same physical and chemical properties as natural compounds, and the Raman signal of deuterated methyl is also at 2100cm -1 or so, so the d of formula Ⅲ 3 -Ac 4 ManNAz,, was used to detect the dynamic expression of natural sugars.
[0041]
[0042] Formula III
[0043] The specific detection method is similar to Example 2, the difference is that the unnatural monosaccharide in the culture medium is d 3 -Ac 4 ManNAz. For specific test results, please refer to Figure 5 and Figure 6 . From Fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com