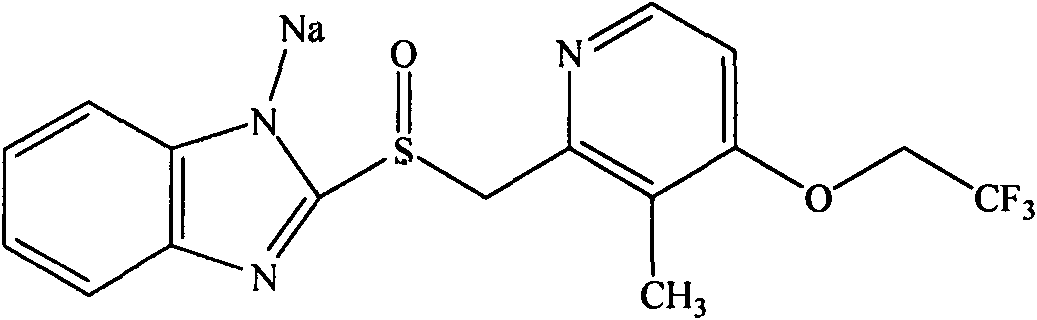
Lansoprazole freeze-dried powder injection and preparation method thereof
A technology of lansoprazole and lyophilized powder, applied in the field of biomedicine, can solve problems such as insufficient formation of excipients, incomplete appearance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
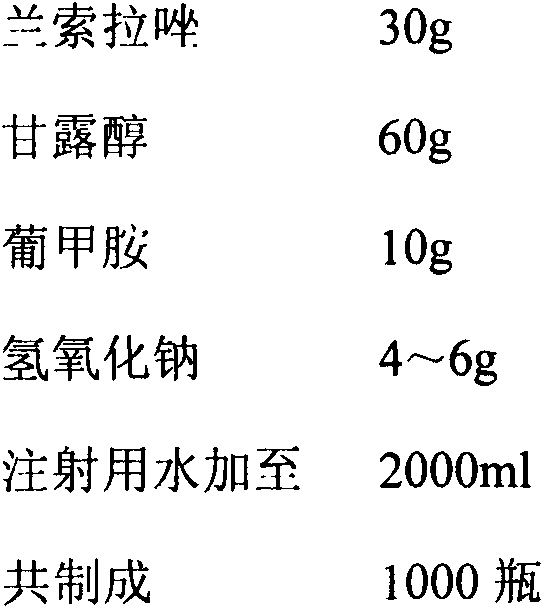
[0029] Ordinary method prepares lansoprazole freeze-dried powder injection (30mg specification)
[0030] prescription
[0031]
[0032] Preparation method: Weigh the prescription amount of raw and auxiliary materials and put them in a medicine barrel, add the prescription amount of water for injection cooled to below 30°C, stir to dissolve completely, add sodium hydroxide to adjust the pH of the liquid medicine to 11.5, add 0.1% (w / v) The required amount of activated carbon for needles is stirred and adsorbed at room temperature for 15 minutes, filtered for decarbonization, sterilized and filtered with a 0.22 μm microporous membrane, the pH and content of the semi-finished product are tested, filled, put into a freeze dryer, and freeze-dried.
[0033] Pre-freezing: first lower the temperature of the lyophilizer plate to below -30°C, and keep it warm for 1h to 2h; freeze-drying: turn on the vacuum pump to evacuate to 0.02MPa, cool the hydrazine to below -50°C, and start lyop...
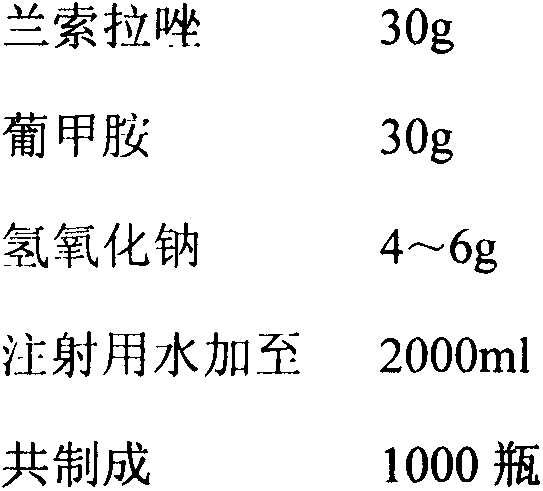
Embodiment 2
[0035] prescription
[0036]
[0037]Preparation method: Weigh the prescription amount of raw and auxiliary materials and put them in a medicine barrel, add the prescription amount of water for injection cooled to below 30°C, stir to dissolve completely, add sodium hydroxide to adjust the pH of the liquid medicine to 11.5, add 0.1% (w / v) The required amount of activated carbon for needles is stirred and adsorbed at room temperature for 15 minutes, filtered for decarbonization, sterilized and filtered with a 0.22 μm microporous membrane, the pH and content of the semi-finished product are tested, filled, put into a freeze dryer, and freeze-dried.
[0038] Pre-freezing: first lower the temperature of the lyophilizer plate to below -30°C, and keep it warm for 1h to 2h; freeze-drying: turn on the vacuum pump to evacuate to 0.02MPa, cool the hydrazine to below -50°C, and start lyophilization. First, heat up to -15°C at a uniform speed for 3 to 5 hours, and keep at -15°C for 5 to...
Embodiment 3
[0040] prescription
[0041]
[0042] Preparation method: Weigh the prescription amount of raw and auxiliary materials and put them in a medicine barrel, add the prescription amount of water for injection cooled to below 30°C, stir to dissolve completely. Add sodium hydroxide to adjust the pH of the medicinal solution to 11.5, add 0.1% (w / v) activated carbon for needles, stir and adsorb at room temperature for 15 minutes, filter for decarbonization, sterilize and filter with a 0.22 μm microporous membrane, and detect the pH and content of the semi-finished product. Fill it, put it into a freeze dryer, and freeze-dry it.
[0043] Pre-freezing: first lower the temperature of the freeze dryer plate layer to 0°C and keep it warm for 30min-1h; then drop it to -5°C and keep it warm for 30min-1h; then drop it down to -10°C and keep it warm for 30min-1h; ℃~-50℃, keep warm for 1h~2h;
[0044] Freeze-drying: Turn on the vacuum pump to evacuate to 0.02MPa, cool the hydrazine down to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com