Preparation method for 2,2-dimethylol alkanoic acid
A technology of dimethylolalkanoic acid and dimethoxyalkylnitrile, which is applied in the field of preparation of 2,2-dimethylolalkanoic acid, can solve the problem of large amount of waste water, low conversion rate of raw materials, and difficulty in product purification, etc. problem, to achieve the effect of high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
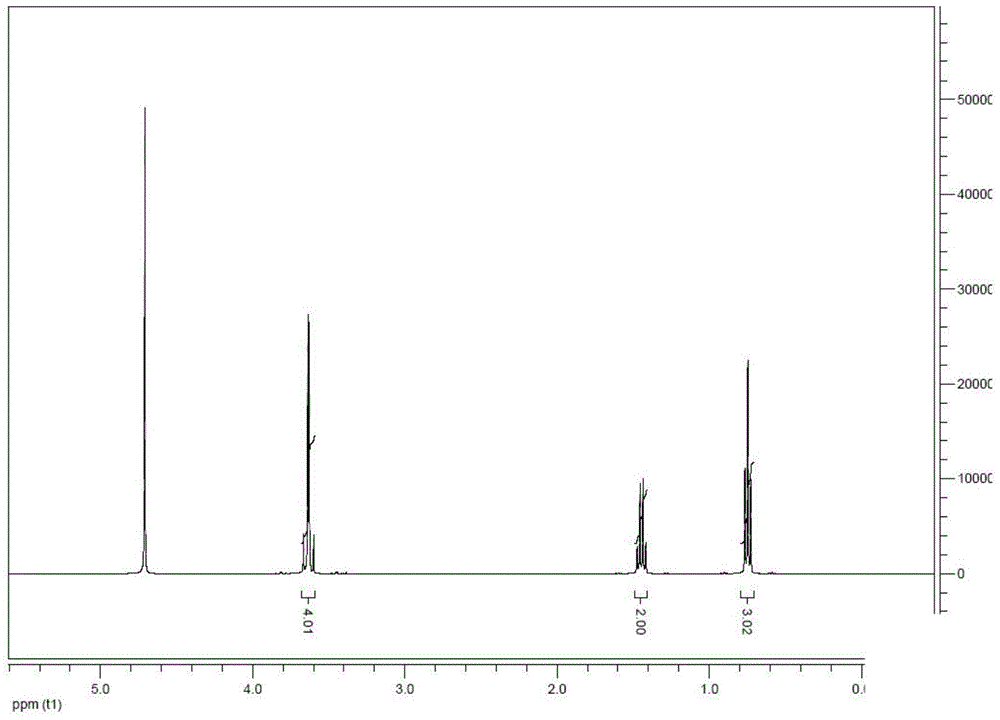
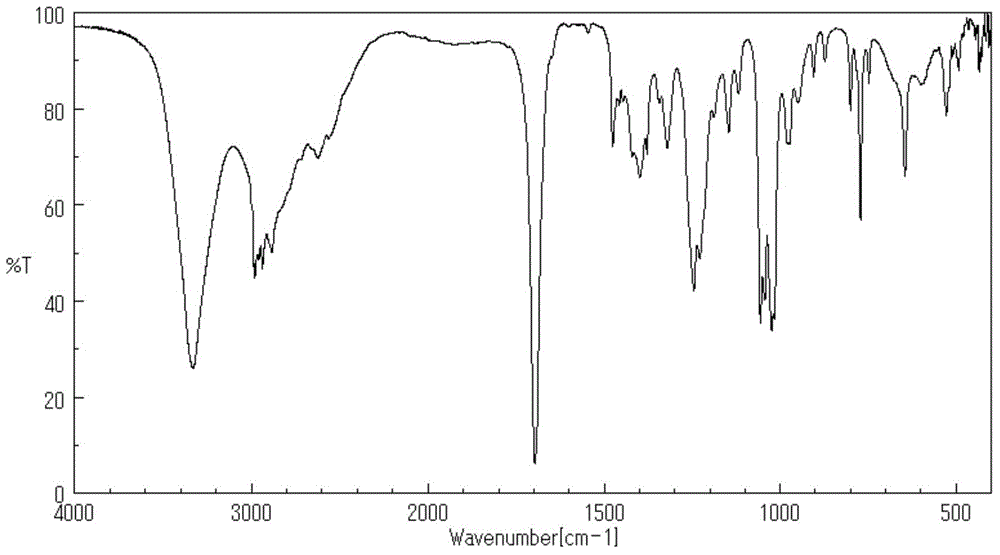
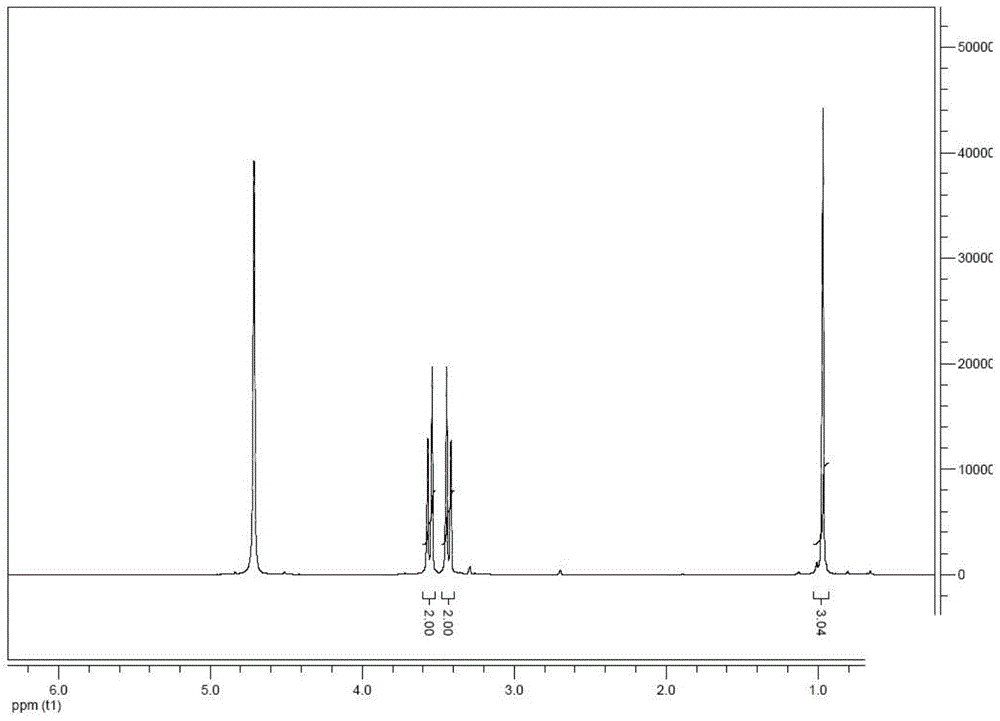
Image
Examples
Embodiment 1
[0048] Dissolve 207g (3.0mol) of n-butyronitrile in 650mL (1040g) of carbon tetrachloride in a 2L three-necked flask equipped with a stirrer, reflux tube, and thermometer, add 246g (6.15mol) of NaOH solid, start stirring and preheat to 50 °C; keep the temperature and add 492 g (6.15 mol) of chloromethyl methyl ether dropwise. After the dropwise addition, the temperature of the reaction system is increased to 80 °C, and the system is kept under reflux for 12 hours.
[0049] After the reaction was finished, excess NaOH and the NaCl produced by the reaction were removed by suction filtration, and the obtained solution was distilled off under reduced pressure to obtain 480 g of concentrated solution. The concentrated solution was dissolved in 250 g of methanol, and 40 wt% of H was added dropwise under agitation. 2 SO 4 Aqueous solution 551.3g (2.25mol), after completion, heated to 70°C for hydrolysis for 5h, after cooling the hydrolyzed solution, 120g of 50wt% NaOH aqueous solutio...
Embodiment 2
[0054] Dissolve 100g (1.8mol) propionitrile in 200mL (180g) tetrahydrofuran (THF) in a 1L three-neck flask equipped with a stirrer, reflux tube, thermometer and inert gas protection, add 423.4g (3.8mol) potassium tert-butoxide at room temperature ), add an ice-water bath, and add 302g (3.8mol) of chloromethyl methyl ether dropwise under full stirring. After the dropwise addition, keep at 0-5°C for 0.5h, remove the ice bath and start heating, and the system is heated to 60°C Reaction 6h.
[0055] After the reaction, the temperature of the system was lowered to room temperature, and H was added dropwise. 2 O6.5g quenches excessive potassium tert-butoxide, stirs 0.2h after adding, adds H 2 0400g dilutes the reaction system, extracts with n-propyl acetate 2 * 350mL, removes the solvent after the organic phase is merged, obtains 280g of viscous concentrated solution, this concentrated solution is dissolved with 140g methanol, drips the H of 40wt% under stirring condition 3 PO 4 ...
Embodiment 3
[0060] Dissolve 100g (1.8mol) of propionitrile in 300mL (280g) of DMF in a 1L three-neck flask equipped with a stirrer, reflux tube, thermometer and inert gas protection, and add 205.2g (3.8mol) of sodium methoxide at room temperature. , add an ice-water bath, add 304g (3.8mol) of chloromethyl methyl ether dropwise under full stirring. After the dropwise addition, keep at 0-5°C for 0.5h, remove the ice bath and start heating to 80°C for 3h.
[0061] After the reaction, the temperature of the system was lowered to room temperature, and H was added dropwise. 2 O6.5g quenches excess sodium methoxide, stirs 0.2h after adding, adds H 2 0600g dilutes the reaction system, extracts with n-propyl acetate 2 * 300mL, removes the solvent after the organic phase is combined, obtains the viscous concentrated solution 265g, and this concentrated solution is dissolved with 120g methanol, drips the HCl aqueous solution 361g of 20wt% under stirring condition, After completion, it was heated to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com