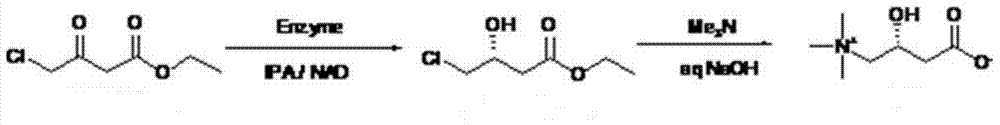
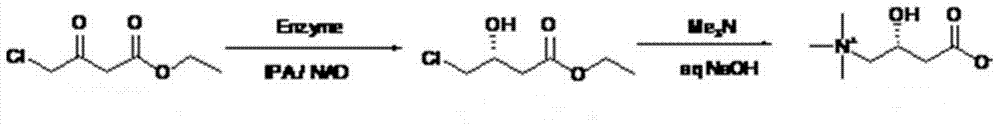
Synthetic method for L-carnitine
A technology of L-carnitine and synthesis method, which is applied in the field of medicine, can solve the problems of high solvent use and production cost, lack of market competitiveness of products, low conversion rate and yield, etc., to improve market competitiveness, reduce production steps, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 ketoreductase
[0029] Inoculate a single colony of recombinant Escherichia coli containing the ketoreductase gene from a glycerol tube or transformation plate into 4 mL of liquid LB medium containing ampicillin resistance and activate overnight (35°C, 220rpm). Transfer 50 mL of liquid LB medium containing ampicillin resistance from the overnight culture at 1 / 100 inoculum, culture at 35°C and 220 rpm until OD 600 When the value reaches 0.6-0.8, add IPTG and continue culturing overnight at 30°C. Cells were collected by centrifugation, and suspended in 10 mL of phosphate buffer (2 mM, pH 7.0). The cell suspension was ultrasonically broken in an ice bath for 15 minutes, centrifuged, the supernatant was pre-frozen overnight, and freeze-dried for 24-40 hours to obtain ketoreductase in the form of freeze-dried powder.
Embodiment 2
[0031] 1) Add ethyl 4-chloroacetoacetate (1.5g, 9.11mmol) into a 50mL round bottom flask, add 20mL phosphate buffer (0.1M, pH6.5), then add 3mL isopropanol and 45mg ketone in sequence Reductase powder and 4.5mg NAD + The dry powder was stirred magnetically at 30°C to start the reaction; at the same time, the pH was maintained at 6.5 using a titrator. GC monitoring, after 30 hours of reaction, the conversion rate was greater than 99%, and the reaction was terminated for use.
[0032] 2) Sodium hydroxide (0.73g, 18.2mmol) and trimethylamine aqueous solution (1.44mL, 10.9mmol) with a mass ratio of 25% were prepared as a mixed solution, and slowly added dropwise to the above-mentioned enzymatic reaction at 0-5°C In the aqueous solution, the addition was completed in about 3 hours, and the stirring reaction was continued at 0-5°C for 24 hours. After the conversion rate was detected by HPLC >99%, the reaction stopped. Add about 3.68mL of hydrochloric acid (concentration: 36%) to n...
Embodiment 3
[0034] 1) Add ethyl 4-chloroacetoacetate (5g, 30.35mmol) to a 100mL round bottom flask, add 40mL phosphate buffer (0.1M, pH6.5), then add 5mL isopropanol and 100mg ketone to reduce Enzyme powder and 10mg NAD + Dry powder, stir at 30°C to start the reaction; at the same time, use a titrator to maintain the pH at 6.5. GC monitoring, after 48 hours of reaction, GC detected that the conversion rate was greater than 99%, and the reaction was terminated for use.
[0035] 2) Sodium hydroxide (2.43g, 60.7mmol) and trimethylamine aqueous solution (21.3mL, 36.12mmol) with a mass ratio of 25% were prepared as a mixed solution, and slowly added dropwise at 0-5°C to the above enzyme-reacted In the aqueous solution, the addition was completed in about 3 hours, and the stirring reaction was continued at 0-5°C for 24 hours. After the conversion rate was detected by HPLC >99%, the reaction stopped. About 12.3 mL of hydrochloric acid (concentration: 36%) was added to neutralize to pH = 6. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com