N-substituted imidazolecarboxylic acid ester chiral compound containing ether side chain, preparation method and application thereof
A technology of imidazole carboxylate and chiral compounds, applied in the field of N-substituted imidazole carboxylate chiral compounds, can solve the weak 11-β hydroxylase binding ability of imidazole derivatives, strengthen the combination of drug molecules and enzyme molecules And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
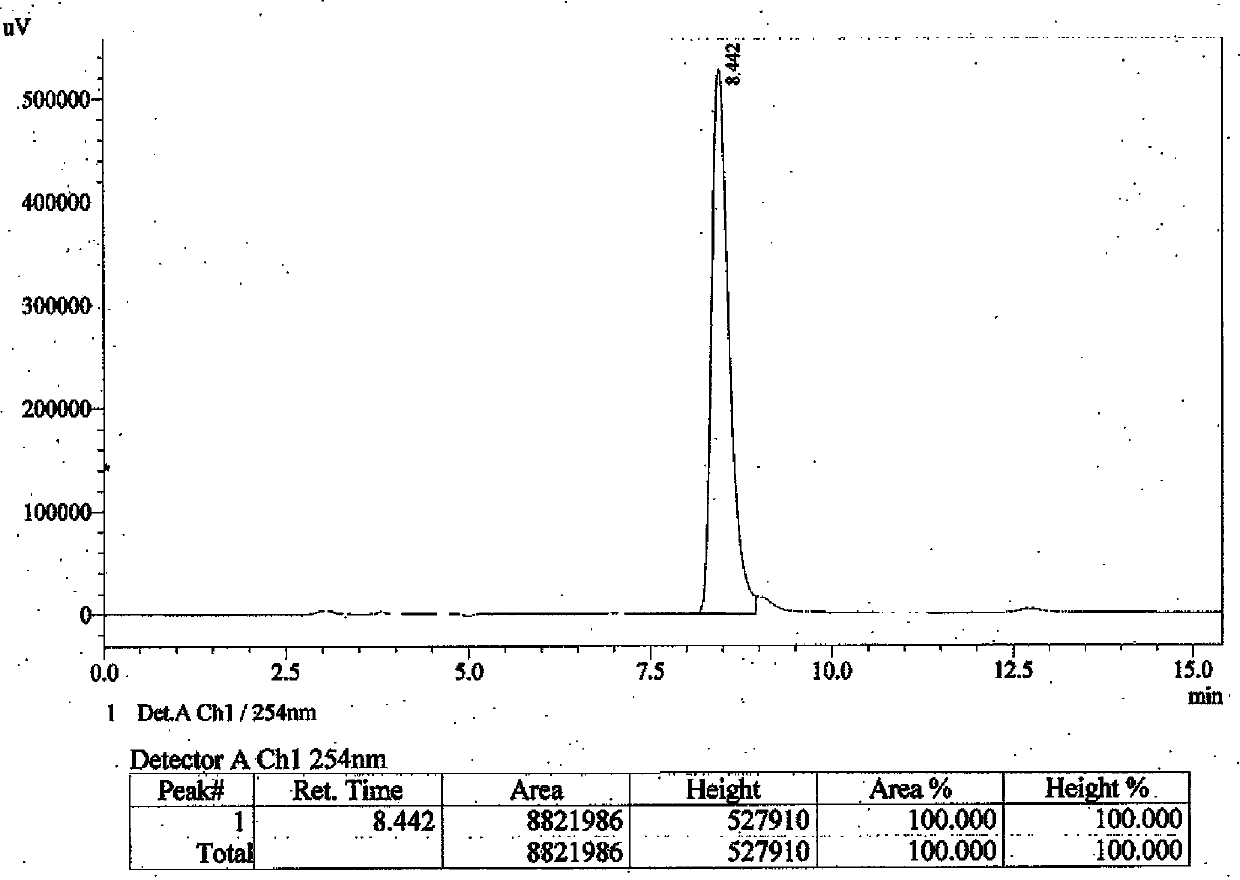
Embodiment 1
[0023] Preparation of the compound of formula (I) of the present invention: compound of formula (II) (CAS: 56649-48-0) (216 mg, 1 mmol), compound of formula (III) (CAS: 6482-24-2) (278 mg , 2 mmol) and anhydrous potassium carbonate (564 mg, 3 mmol) were mixed in 15 mL N,N-dimethylformamide, and the mixture was stirred at 50°C overnight. The next day, the reaction solution was poured into 100 mL of cold water to obtain a clear solution, extracted 3 times with ethyl acetate (50 mL each time), the organic layers were combined, dried with anhydrous sodium sulfate overnight, filtered to obtain the filtrate, and the solvent was evaporated to dryness under reduced pressure The oily crude product was obtained, which was purified by a silica gel column (developing solvent: cyclohexane / ethyl acetate=3 / 2) to obtain 150 mg of a colorless oily product with a yield of 54%.
[0024] 1) NMR: Instrument: BRUKER, TMS as internal standard.
[0025] 1 H-NMR (400MHz CDCl 3 ) δ : 1.862 (3H, d,...
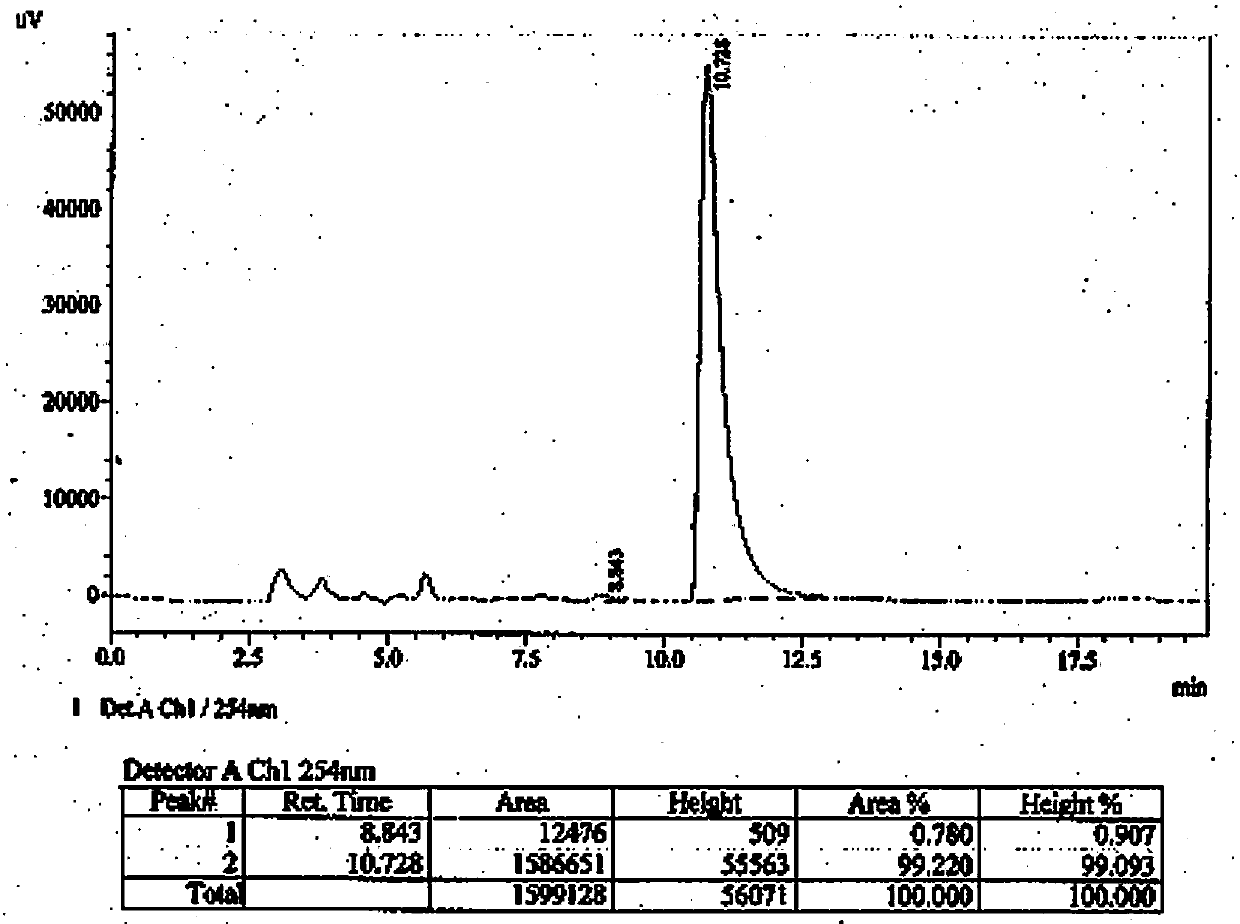
Embodiment 2
[0032]Preparation of the compound of formula (I) of the present invention: compound of formula (II) (CAS: 56649-48-0) (216 mg, 1 mmol), compound of formula (III) (CAS: 627-42-9) (188 mg , 2 mmol) and anhydrous potassium carbonate (564 mg, 3 mmol) were mixed in 15 mL N,N-dimethylformamide, and the mixture was stirred at 50°C overnight. The next day, the reaction solution was poured into 100 mL of cold water to obtain a clear solution, extracted 3 times with ethyl acetate (50 mL each time), the organic layers were combined, dried with anhydrous sodium sulfate overnight, filtered to obtain the filtrate, and the solvent was evaporated to dryness under reduced pressure The oily crude product was obtained, which was purified by a silica gel column (developing solvent: cyclohexane / ethyl acetate=3 / 2) to obtain 120 mg of a colorless oily product with a yield of 43%.
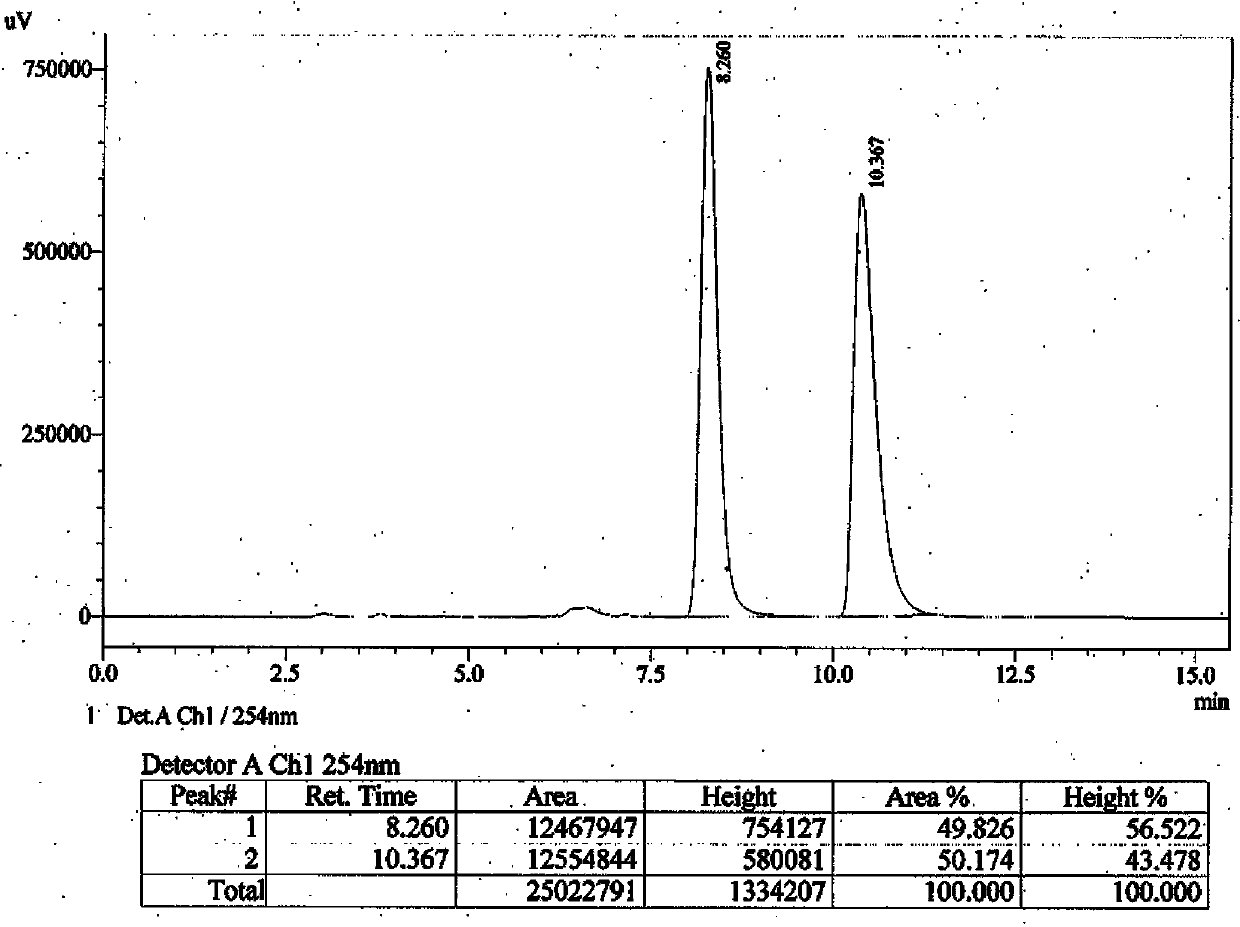
Embodiment 3
[0034] Preparation of S-type optical counterpart compound (Ⅳ):
[0035] S-(-)-1-(1-phenylethyl)-1-hydro-imidazole-5-carboxylic acid (CAS: 56649-49-1) 216 mg, 1 mmol), formula (Ⅲ) compound (CAS: 627-42-9) (188 mg, 2 mmol) and anhydrous potassium carbonate (564 mg, 3 mmol) were mixed in 15 mL N,N-dimethylformamide, and the mixture was stirred at 50°C overnight. The next day, the reaction solution was poured into 100 mL of cold water to obtain a clear solution, which was extracted three times with ethyl acetate (50 mL each time), the organic layers were combined, dried over anhydrous sodium sulfate, filtered to obtain the filtrate, and the solvent was evaporated to dryness under reduced pressure The oily crude product was obtained, which was purified by a silica gel column (developing solvent: cyclohexane / ethyl acetate=3 / 2) to obtain 170 mg of a colorless oily product, with a yield of 61.2%.
[0036] 1) NMR: Instrument: BRUKER, TMS as internal standard.
[0037] 1 H-NMR (400MH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com