Human anti-thrombin III heparin combination ratio detection method
An antithrombin and detection method technology, applied in the field of biomedical detection and analysis, can solve the problems of high price, low accuracy of calculation results, complicated and difficult operation, etc., achieve high accuracy and accuracy of results, eliminate human interference, sample Handling simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
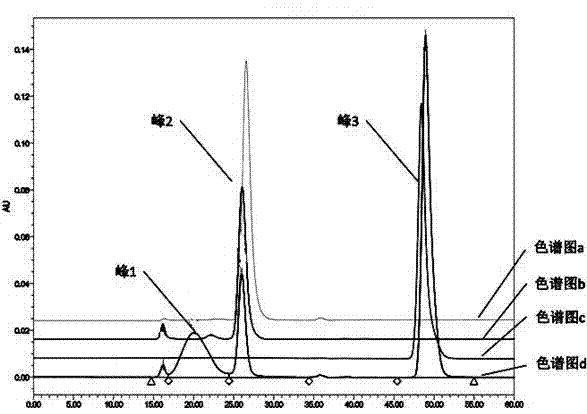
Image
Examples
Embodiment 1
[0047] 1. Reagents and samples are shown in Table 1:
[0048] Table 1
[0049] .
[0050] 2. Solution preparation:
[0051] Phosphate buffer: Weigh 12.0 g of sodium dihydrogen phosphate, dissolve it in 950 ml of purified water, adjust the pH to 7.4 with 0.2 mol / L sodium hydroxide solution, dilute to 1 L with purified water, and mix well.
[0052] Mobile phase: Take 495ml of the above-mentioned phosphate buffer solution, add 5ml of isopropanol, mix well and ultrasonicate for 30min for degassing.
[0053] Heparin solution: dilute heparin sodium injection to 5000IU / ml with the above mobile phase.
[0054]Inactivated human antithrombin III: add Bicine solution (50mmol / L Bicine (N-di(hydroxyethyl)glycine), 100mmol / L NaHCO3, pH 8.3) to human antithrombin III standard ; Then add 10~30mmol / L trinitrobenzenesulfonic acid, and treat at 25°C for 30min; add 20 times the volume of Tris buffer (50mmol / LTris–HCl, 100mmol / L NaCl, pH7.4) and mix well. After concentration by ultrafiltrat...
Embodiment 2
[0078] 1. Reagents and samples are shown in Table 5:
[0079] table 5
[0080] .
[0081] 2. Solution preparation:
[0082] Phosphate buffer: Weigh 20.0 g of sodium dihydrogen phosphate, dissolve it in 950 ml of purified water, adjust the pH to 6.4 with 0.2 mol / L sodium hydroxide solution, dilute to 1 L with purified water, and mix well.
[0083] Mobile phase: Take 495ml of the above-mentioned phosphate buffer solution, add 5ml of isopropanol, mix well and ultrasonicate for 30min for degassing.
[0084] Heparin solution: Dilute heparin sodium injection to 2000IU / ml with the above mobile phase.
[0085] Inactivated human antithrombin III: add Bicine solution (50mmol / L Bicine (N-bis(hydroxyethyl)glycine), 100mmol / L NaHCO) to human antithrombin III standard 3 , pH 8.3); then add 10~30mmol / L trinitrobenzenesulfonic acid, and treat at 25°C for 30min; add 20 times the volume of Tris buffer (50mmol / LTris–HCl, 100mmol / L NaCl, pH7.4) Mix well. After concentration by ultrafiltra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com