Targeting anti-tumor fusion protein, and encoding gene and expression plasmid thereof
A technology of fusion protein and coding gene, which is applied in the field of medicine and biology, can solve the problems of constructing fusion protein without AnnexinA5 and melittin, complex pharmacological effects, and limited application, so as to achieve the effect of improving killing effect, reducing drug dosage, and reducing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Obtaining of fusion gene mAnxA5-MLT
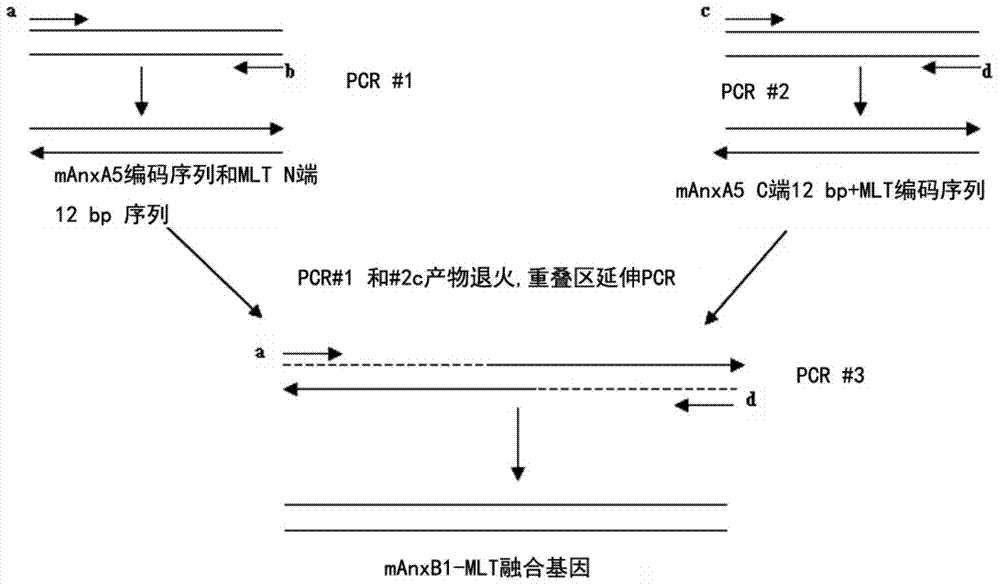
[0074] See figure 1 , the gene encoding annexin mAnxA5 and the gene encoding melittin were respectively amplified by PCR; and then connected by overlapping region extension method to obtain fusion gene pET28a-mAnxA5-MLT. Specific steps are as follows:
[0075] (1) Use primer a and primer b to amplify the entire coding gene of mAnxA5 gene. Wherein primer a is 5'GCG GAATTC ATGGCCTACTGTCGCTCCCT3', the primer is consistent with the 5' end of the annexin B1 coding sequence, and introduces an EcoR I restriction endonuclease site. Primer b: 5'TTGAAACTCATCGGCCCTGCA3'. PCR reactions were performed using high-fidelity pfu DNA polymerase with a dNTP concentration of 20 μM, Mg 2+ The concentration was 1.5 mM, the temperatures of denaturation, annealing, and extension were 94°C, 55°C, and 72°C, and the times were 45s, 45s, and 75s, respectively, and a total of 30 cycles were performed.
[0076] (2) Use primers c and d to amplif...
Embodiment 2
[0079] Example 2 Construction of expression plasmid pET28a-mAnxA5-MLT
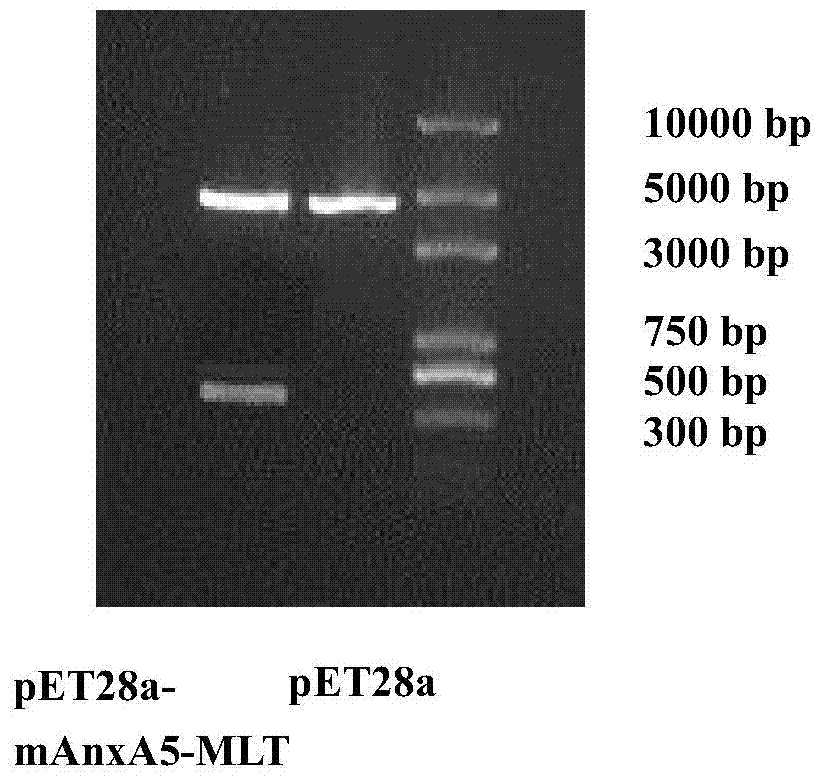
[0080] The fusion gene mAnxA5-MLT amplified by the above method was sequenced using primers a and d (SEQ ID NO:3). After the sequencing was correct, it was digested with EcoR I and Sal I, and connected with the vector pET-28a (+) which was digested with EcoR I and Sal I. Restriction enzymes and T for digestion and ligation reactions 4 All ligases were purchased from TaKaRa Company, and the reaction was carried out using the system recommended by the enzyme instructions.
[0081] The inducible expression vector pET-28a (+) used in the present invention has a full length of 5369bp and contains a T7 promoter and a plasmid origin of replication (ori).
[0082] The specific reaction process is as follows: use EcoR I and Sal I to perform double enzyme digestion on the above PCR product and vector, and use a high-salt (H) buffer system for the reaction, and digest at 37°C for 3 hours. After separation by 1.0% ...
Embodiment 3
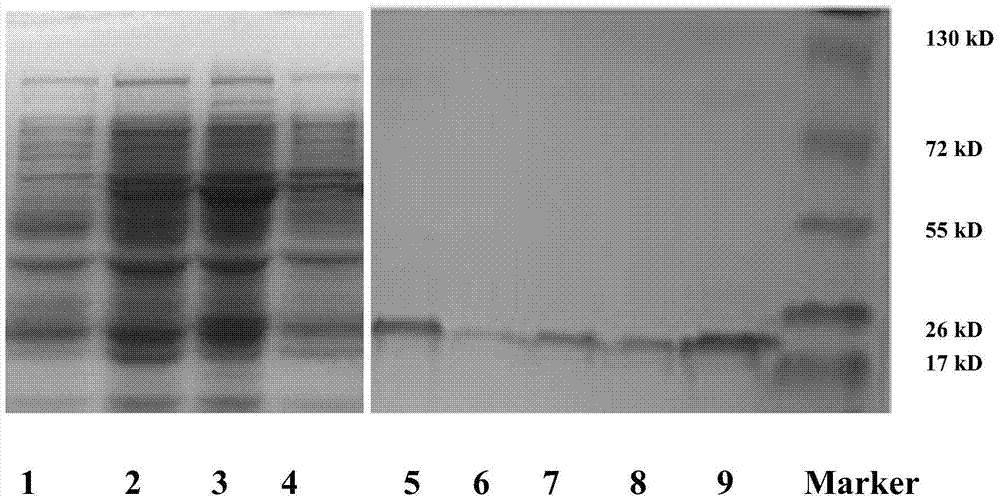
[0083] Embodiment 3 Preparation of engineering bacteria
[0084] The above ligated product was transformed into Escherichia coli strain BL21(DE3) to obtain engineering strain BL21 (pET28a-mAnxA5-MLT).
[0085] The specific steps are: first culture the host strain BL21 in 50ml LB medium (containing 1% tryptone, 0.5% yeast extract and 1% sodium chloride, pH 7.0) at 37°C until OD 600 At 0.4, centrifuge at 4000rpm at 4°C to collect the host bacteria; remove the supernatant and resuspend with 1-2ml ice-cooled 100mmol / L calcium chloride solution to obtain competent bacteria. Cool 100 ng of the plasmid constructed above and competent bacteria in an ice bath for 30 minutes, heat shock at 42° C. for 90 seconds, and then cool in an ice bath for 10 minutes. Add 0.8ml of LB, incubate at 37°C for 1 hour, take 0.2ml of the transformation product and smear it on an LB agar plate, and incubate at 37°C for 12-15 hours to obtain a single-clonal host bacterium containing the recombinant plasmid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com