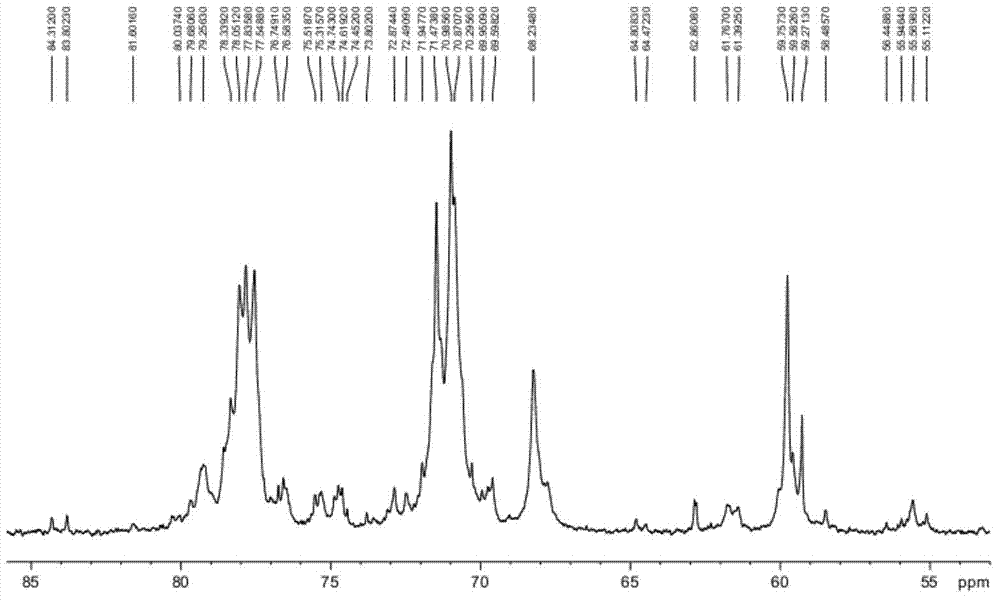
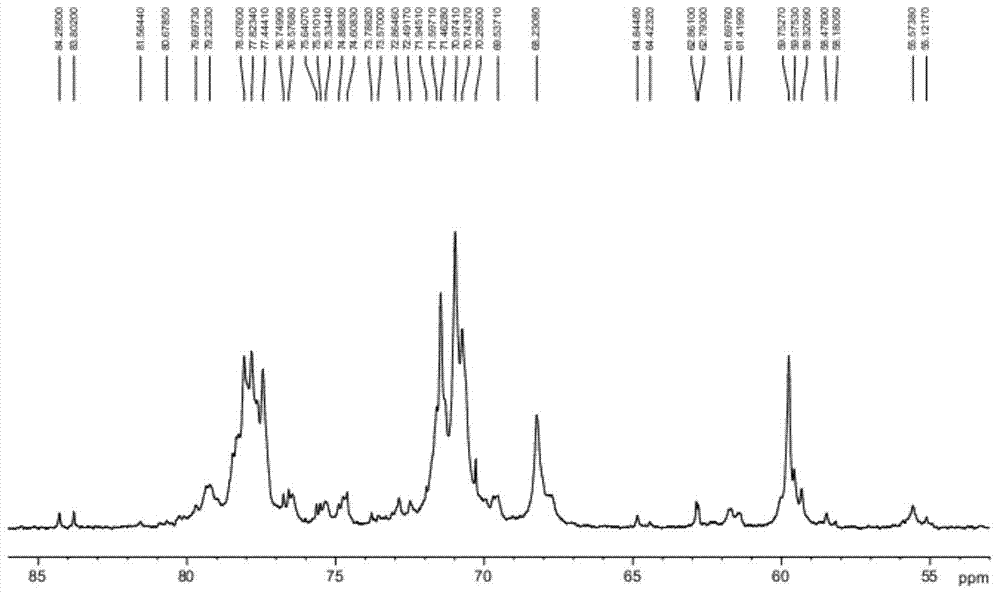
Preparation method of heparin sodium
A technology of heparin sodium and sodium carbonate, which is applied in the preparation of heparin sodium and the field of heparin sodium, can solve the problems of heparin destruction, adverse effects of industrial production, and lack of conditions for industrial production, and achieve stable properties of heparin, not easy to change color, and high potency high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Impurity removal: add crude heparin sodium (potency ≥ 50IU / mg, its 0.01% (g / mL) aqueous solution has an optical absorption value ≤ 1.0 at a wavelength of 260nm) in an amount of 20% (g / mL) In purified water, warm up to 30°C and stir until completely dissolved. Then add sodium chloride in an amount of 5% (g / mL), adjust the pH of the solution to 10.0-10.5 after the sodium chloride is completely dissolved, stir at a constant temperature for 4.0 hours, then raise the temperature to 85°C, and maintain the pH of the solution at 9.5-10.0 Keep it for 10 minutes, then quickly cool the feed liquid to 15°C, let it stand for 5 hours and filter it with a clarification plate until the feed liquid is clear, and take the filtrate;
[0045] (2) Primary enzymatic hydrolysis: raise the temperature of the above feed solution to 50°C, then adjust the pH to 9.0 with 18% (g / mL) hydrochloric acid aqueous solution or 20% (g / mL) sodium hydroxide aqueous solution, and add crude heparin sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com