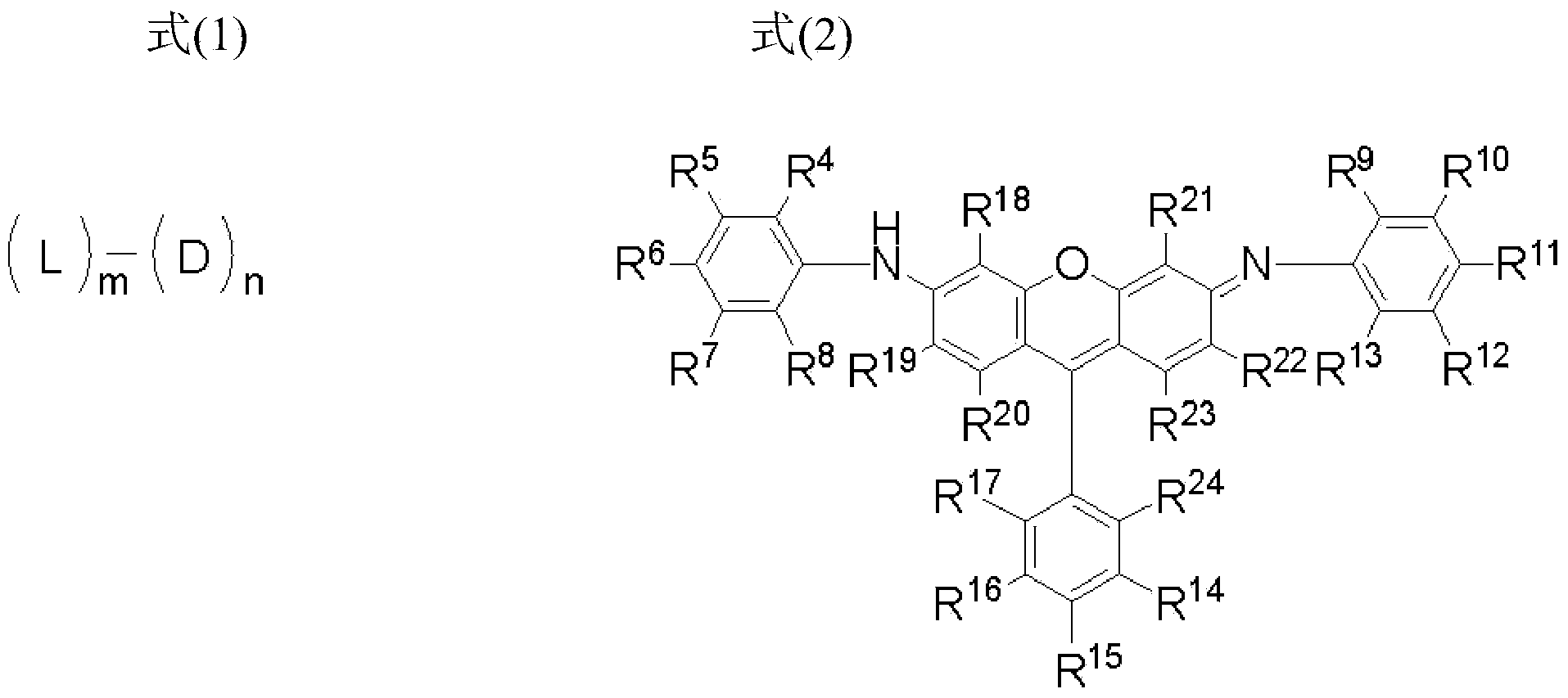
Novel compound having xanthene derivative multimeric structure, colored composition, ink for inkjet recording, inkjet recording method, color filter, and color toner
一种着色组合物、化合物的技术,应用在具有呫吨衍生物的多聚体结构的新型化合物、着色组合物、用于喷墨记录的油墨、喷墨记录、彩色滤光片和彩色调色剂领域,能够解决性能如耐臭氧性和耐光性低等问题,达到耐光性改进的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
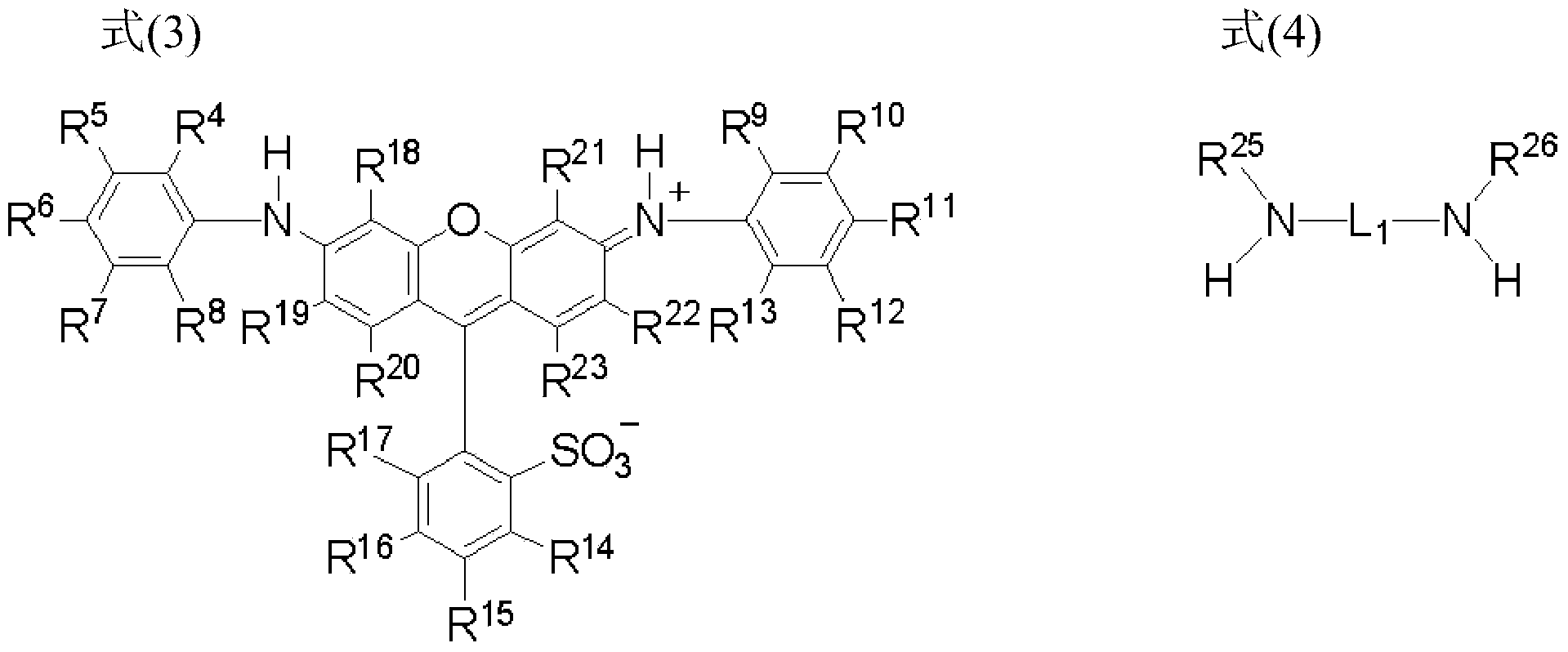
[0147] From the viewpoint of availability of materials and cheap manufacture, it is preferable that the compound represented by formula (1) is a compound synthesized by a synthetic method comprising:
[0148] a step of chlorosulfonylation of the compound represented by formula (3),
[0149] a step of reacting the chlorosulfonylated compound with a diamine compound represented by formula (4), and
[0150] A step of hydrolyzing residual chlorosulfonyl groups.
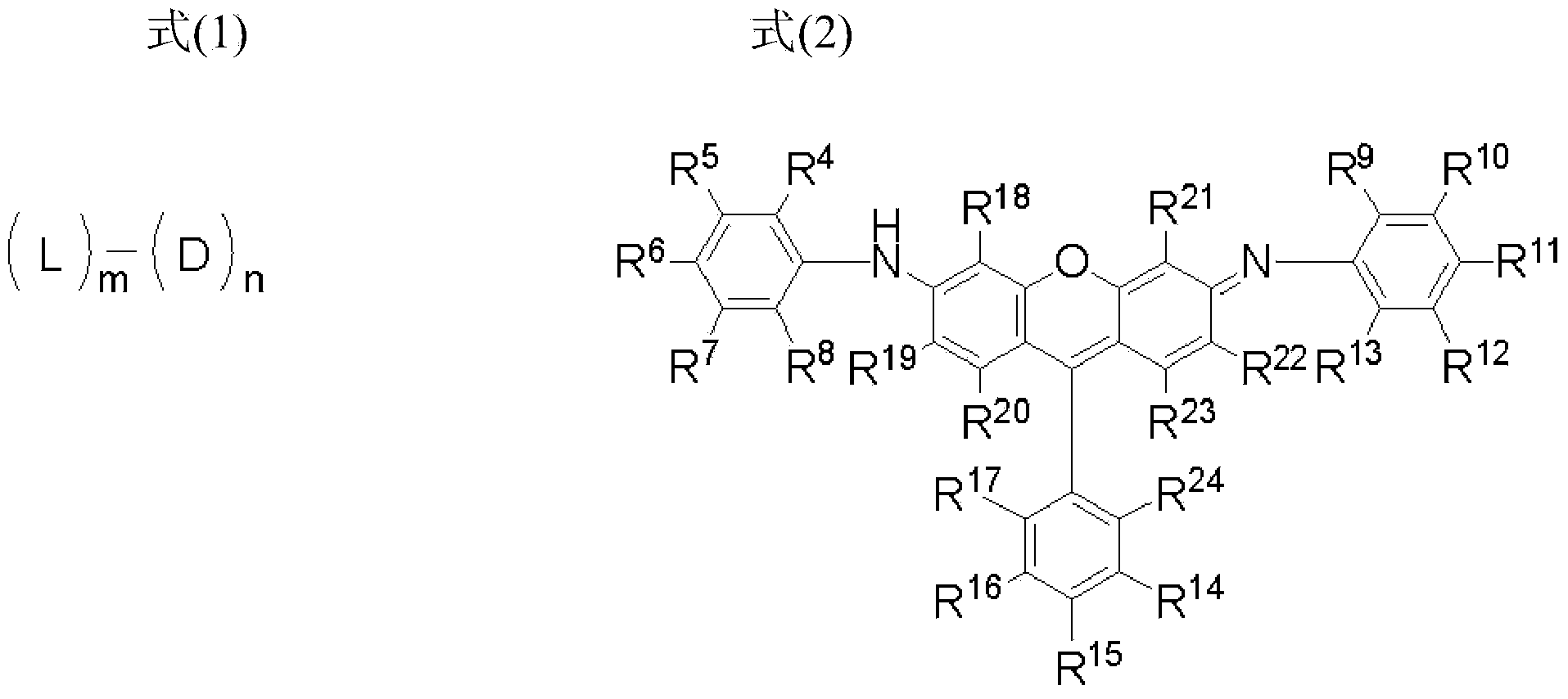
[0151]
[0152] In formula (3), R 4 to R 23 Each independently represents a hydrogen atom or a substituent.
[0153] In formula (4), R 25 and R 26 each independently represents a hydrogen atom or a substituent, and L 1 Indicates a divalent linking group.
[0154] In formula (3), R 4 to R 23 Respectively with R in formula (2) 4 to R 23 The same meaning, and the preferred examples are also the same.
[0155] In formula (4), R 25 and R 26 Each independently preferably represents a hydrogen atom, a substitute...
Embodiment
[0340] (synthesis example)
[0341] The synthesis method of the compound (mixture) of the present invention will be described in detail in Examples, but the present invention is not limited thereto. In the examples, "%" and "parts" refer to mass % and mass parts unless otherwise specified.
[0342] [Synthesis of Exemplary Compound 1]
[0343]
[0344]
[0345] 5.5 g of cyanuric chloride and 2 or 3 drops of sulfated vegetable oil were added to 20 g of ice water and stirred at 10° C. or lower. A solution obtained by dissolving 8.4 g of monosodium aniline-2,5-disulfonate in 30 g of water and a 2N aqueous sodium hydroxide solution were dropped thereinto, keeping the temperature at 10° C. or lower. While maintaining the pH of the reaction solution at 4.5 with 2N sodium hydroxide, after performing the reaction in the reaction system for 2 hours at an internal temperature of 10°C or lower, the insoluble matter (reaction solution) was filtered through a GF / F filter (manufactured ...
Embodiment 2
[0461] [Example 2] Manufacture and evaluation of color toner
[0462]
[0463] 3 parts by mass of the colorant of the present invention (in the exemplary compound (1), m=1, n=2) and 100 parts by mass of the resin for toner [styrene-acrylate copolymer, HIMER TB- 1000F (trade name, manufactured by Sanyo Chemical Industries, Ltd)] mixed and pulverized in a ball mill, then melted and mixed by heating at 150° C., coarsely pulverized using a hammer mill after cooling, and then finely pulverized with an air jet method Finely pulverize. Particles of 1 μm to 20 μm are selected by further classification to prepare a toner.
[0464]
[0465] Carrier iron powder (900 parts by mass) (EFV250 / 400, trade name, manufactured by Nippon Teppun Co., Ltd.) was uniformly mixed with 10 parts by mass of the above toner to prepare a developer. As a result of copying by using the above developer and a dry electronic plain paper copier (NP-5000, trade name, manufactured by Canon Inc), the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com