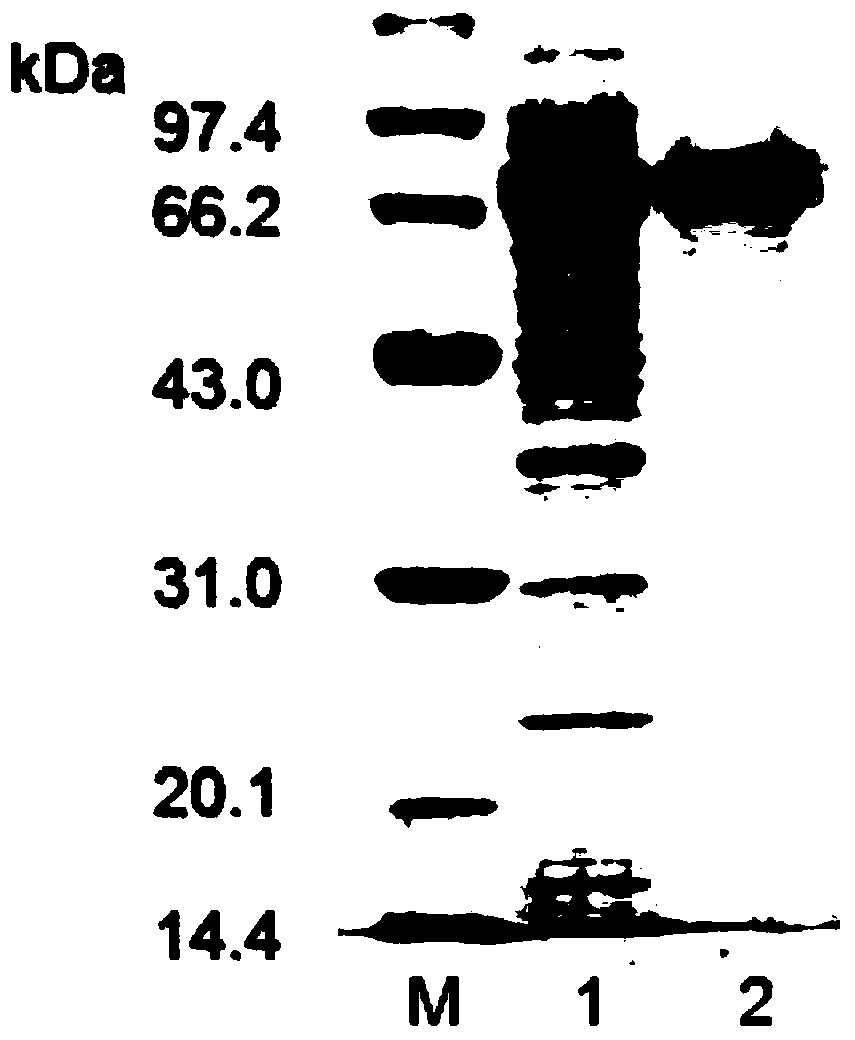L-asparaginase as well as encoding gene and application thereof
A coding gene and coding technology, applied in application, genetic engineering, plant genetic improvement, etc., can solve the problem of lack of characteristic L-asparaginase products, etc., and achieve the effect of reducing the production amount and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the acquisition of L-asparaginase gene
[0048] 1. PCR amplification of the conserved region of the L-asparaginase gene
[0049] (1) According to the amino acid sequence of L-asparaginase reported in GenBank, use the online software BlockMaker (http: / / blocks.fhcrc.org / blocks / blockmkr / make_blocks.html) to search for the conserved region, and then use the online primer design Software CODEHOP (http: / / blocks.fhcrc.org / codehop.html) designed degenerate primers AsnDF (forward primer): 5'-TCGTCCTGCACGGACNGAYACNATG-3', AsnDR (reverse primer): 5'-CGTTGCCGGCGCCAAANGTYTC-3' .
[0050] (2) Genomic DNA of Rhizomucor miehei CAU432 was used as template, AsnDF and AsnDR were used as primers, and Ex Taq DNA polymerase was used to amplify to obtain PCR amplification products.
[0051] PCR reaction conditions: 94°C for 5min; 94°C for 30s, 60°C-55°C (0.5°C for each cycle) for 30s, 72°C for 1min, a total of 10 cycles; 94°C for 30s, 55°C for 30s, 72°C for 1min, a total of 20...
Embodiment 2
[0067] Embodiment 2, expression of recombinant L-asparaginase
[0068] 1. Construction of recombinant strains
[0069] (1) Primer design
[0070] Upstream primer RmAsnAF: 5'-GGGTTT CATATG GATTCGAGAACGACTGCT-3';
[0071] The underlined sequence is the recognition site for Nde I digestion;
[0072] Downstream primer RmAsnAR: 5'-ATTCCG CTCGAG TTCTTTTCCTAGAAGTTGTGC-3';
[0073] The underlined sequence is the Xho I restriction recognition site.
[0074] (2) Extract the mRNA of Rhizomucor miehei CAU432 and reverse transcribe it into cDNA, use the cDNA as a template, and use RmAsnAF and RmAsnAR as primers to carry out PCR amplification to obtain the PCR amplification product, and the agarose gel electrophoresis image of the product Such as figure 1 shown. figure 1 Among them, M is a DNA marker, and 1 is a PCR amplification product.
[0075] PCR reaction conditions: pre-denaturation at 94°C for 5 min; denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 7...
Embodiment 3
[0092] Embodiment 3, the property of recombinant L-asparaginase (RmAsnase)
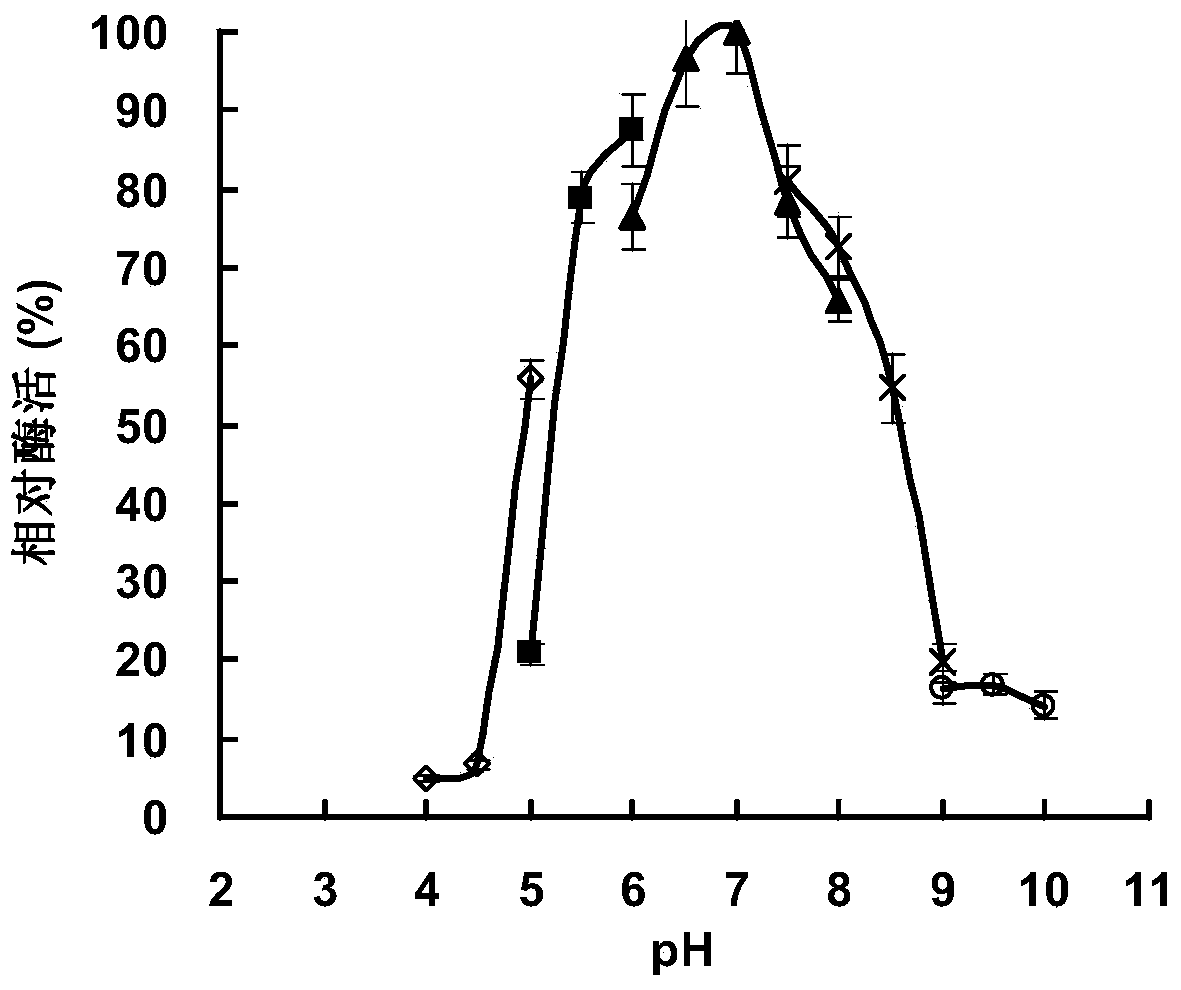
[0093] 1. The optimal reaction pH and pH stability of RmAsnase
[0094] Determination of the optimum reaction pH: the RmAsnase prepared in Example 2 was used as the enzyme solution to be tested, and the enzyme activity of L-asparaginase was measured.
[0095] The only difference lies in the use of the following different buffers (both at a concentration of 50mmol / L): citric acid buffer (pH3.0-6.0), MES (2-(N-morpholine)ethanesulfonic acid) buffer ( pH5.5-6.5), phosphate buffer (pH6.0-8.0), Tris-HCl (trishydroxymethylaminomethane-hydrochloric acid) buffer (pH7.5-9.0), CHES (2-cyclohexylamino ethanesulfonic acid) buffer (pH8.0-10.0). The same amount of RmAsnase was dissolved in the same volume of five buffer systems with different pH values. Then the enzyme activity of L-asparaginase was measured at 45°C, and the highest enzyme activity was taken as 100%, and the relative enzyme activity in each syst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com