Method for directly dissolving uranium dioxide or spent fuel oxides with ionic liquid
An ionic liquid, iron-based ionic liquid technology, applied in reactor fuel elements, radioactive purification, reduction of greenhouse gases, etc., can solve the problems of high operating temperature, strict protection requirements, not meeting the requirements of green chemistry development, etc., and achieve good economy. , the effect of high dissolution efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1[Bmim]FeCl 4 、[Bdmim]FeCl 4 and [Emim]FeCl 4 UO in ionic liquid 2 Dissolution studies of
[0029] [Bmim]FeCl 4 、[Bdmim]FeCl 4 and [Emim]FeCl 4 Pipette 1ml of the ionic liquid into the reaction bottle, and then add 40.3mg of UO 2 , Stir the reaction in an oil bath at 160°C. After reacting for 2 hours, the reaction system still had a black slurry, and after continuing to react for 8 hours, the reaction system still did not become clear. After fully shaking, draw a small amount of reaction solution into a 10ml plastic centrifuge tube, dilute with acetone and centrifuge to separate unreacted UO 2 , and then spin off acetone with HNO at a pH of about 2 3 After diluting to an appropriate concentration, perform ICP-AES test. When drawing the uranium calibration curve, it is necessary to add the same amount of iron-based ionic liquid as the test sample to offset the interference of the matrix. It is found that the uranium concentration in the uranium standard...
Embodiment 2
[0030] Example 2[Bmim]FeCl 4 、[Bdmim]FeCl 4 and [Emim]FeCl 4 Adding its corresponding imidazolium chloride salt to the ionic liquid on 2 Dissolution studies
[0031] In 1.0ml iron-based ionic liquid, add its corresponding imidazolium chloride salt in an equimolar amount, then add 50.2mg UO to the reaction bottle 2 ,, The reaction was carried out in an oil bath at 160°C. After reacting for 2 hours, the reaction system became clear without black insoluble matter. After that, add a certain amount of UO to the reaction bottle every 2h 2 , until the final reaction system has black insoluble matter. Then, the dissolved uranium content was analyzed by ICP-AES according to the same method as above, and the test results showed that after adding imidazolium chloride salt in an equimolar amount to the iron-based ionic liquid, 286 mg of UO could be dissolved in every milliliter of iron-based ionic liquid 2 .
Embodiment 3
[0032] Embodiment 3 Reaction temperature is to UO in the mixed ionic liquid 2 Effect of dissolution
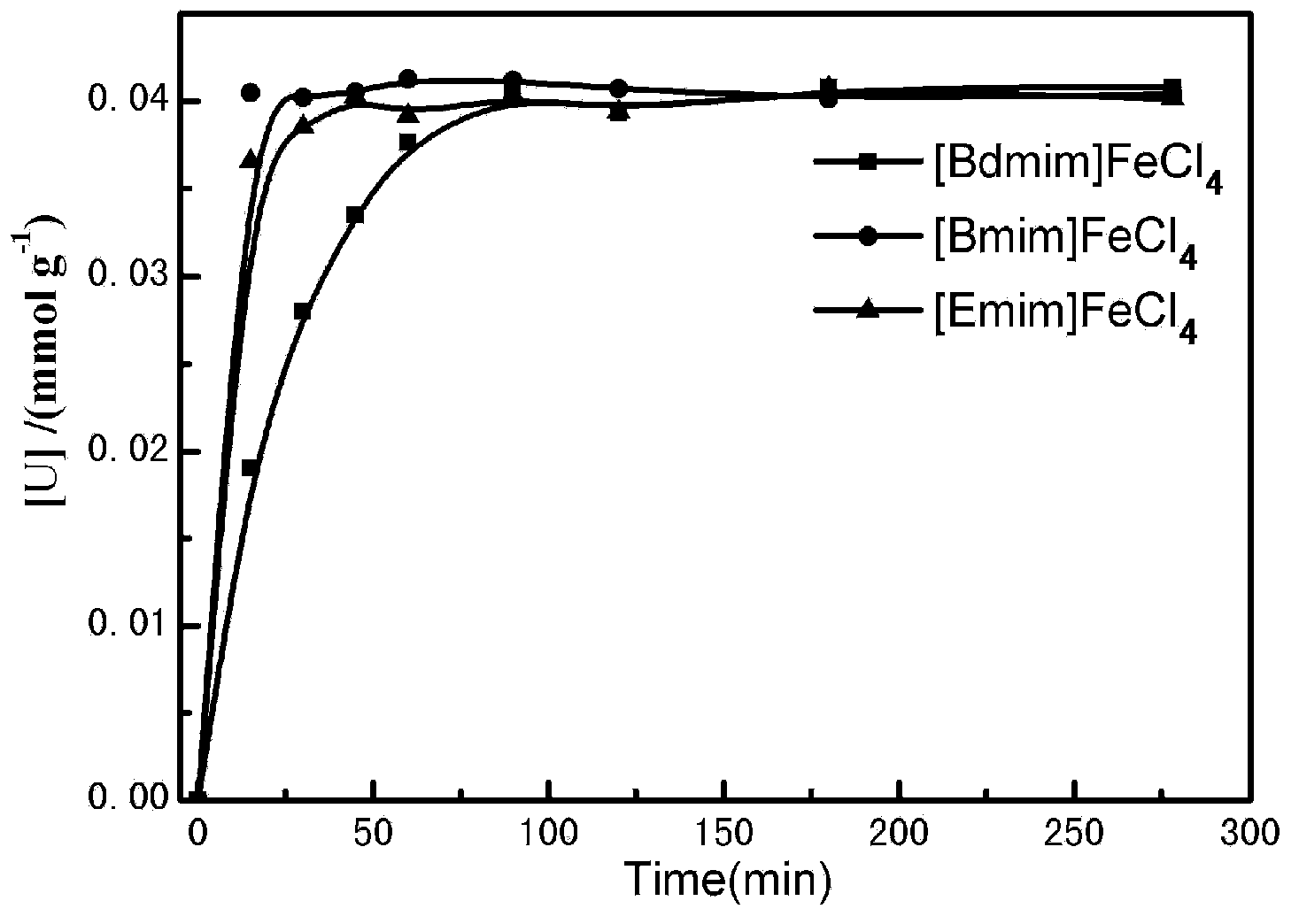
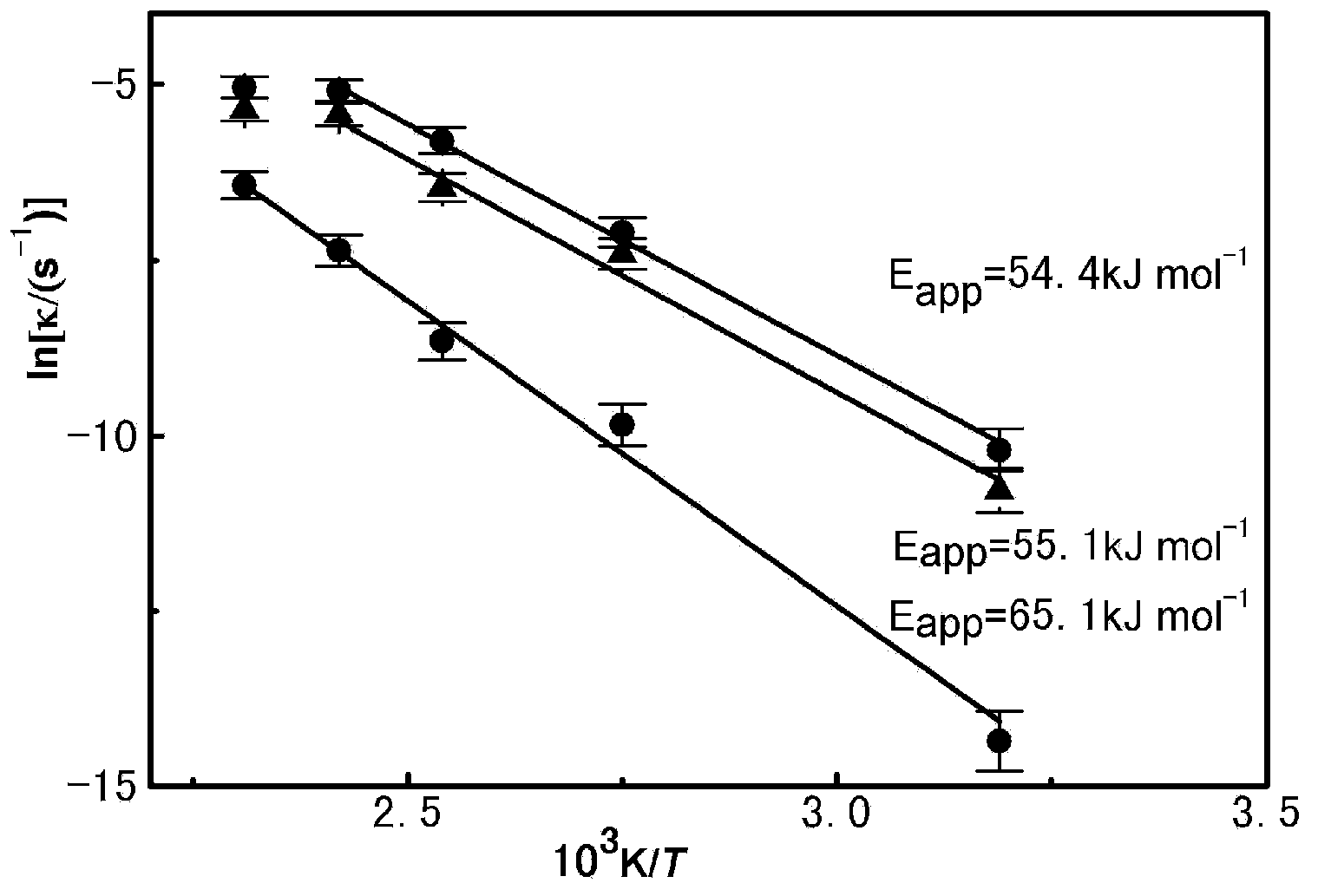
[0033] In 1.0ml of iron-based ionic liquid, add its corresponding imidazolium chloride salt in an equimolar amount, and then add 25.3mg of UO 2 , three mixed ionic liquid systems were reacted in an oil bath at 40, 90, 120, 140 and 160 °C, respectively. Absorb a certain amount of reaction solution from the reaction system at regular intervals to analyze the dissolved uranium content within this time range. The results of the dissolved uranium with time at a reaction temperature of 140°C are shown in the appendix figure 1 ( figure 1 The three curves in the figure represent the mixed system of the iron-based ionic liquid and its corresponding imidazolium chloride salt respectively), and the effect of reaction temperature on the dissolution of uranium is characterized by fitting the apparent activation energy, see appendix figure 2 . The test results showed that [Bdmim]FeCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com