A sesquiterpene compound and its preparation method and use
A technology for sesquiterpenoids and compounds, applied in the field of caryophyllene-type new sesquiterpenoids and their preparation, can solve the problems of unsuitability for industrial production and preparation, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
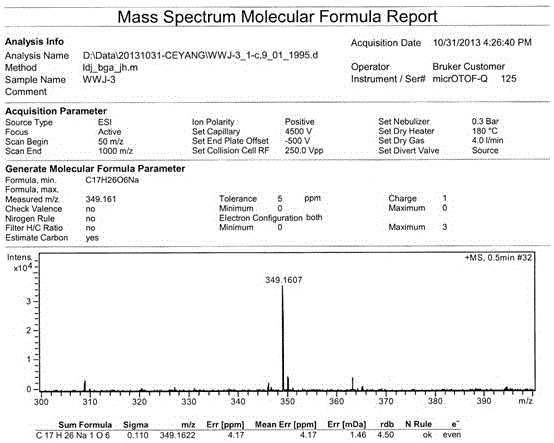
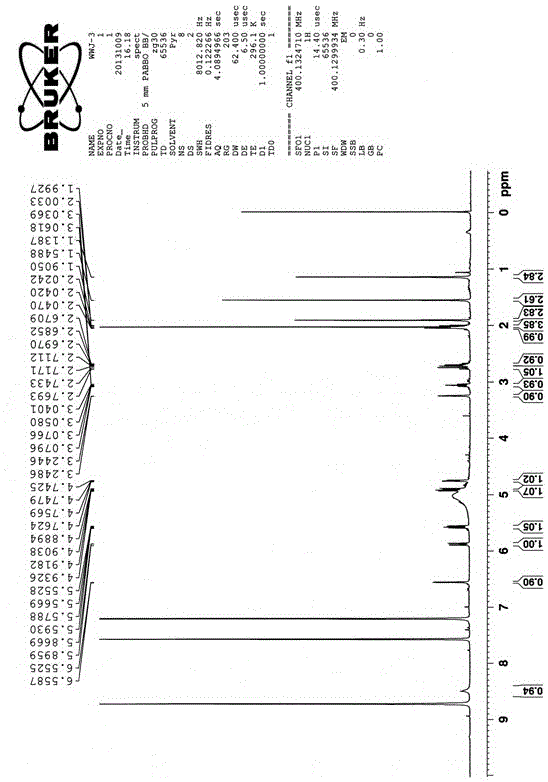
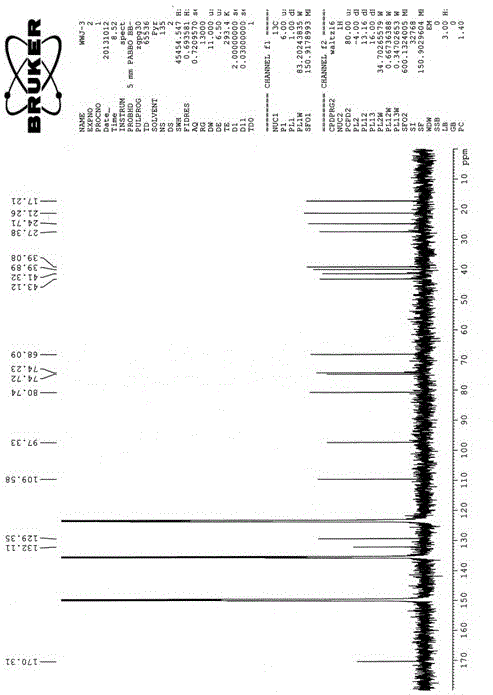
[0043] Example 1: R is H and R is CH 3 Preparation of the compound of formula I:
[0044] Pick the spores of the fungus of the genus Ascotrichasp.ZJ-M-5, the preservation number is CGMCCNo.8278, and directly inoculate them into a liquid culture medium for 7 days on a shaker. The culture medium is prepared by distilled water and contains 3% sucrose , NaNO 3 0.3%, KCl0.05%, FeSO 4 ·7H 2 O0.001%, K 2 HPO 4 0.1%, the culture temperature is 28°C, the shaker speed is 180 rpm, the fermentation broth is filtered to remove the mycelia, the filtrate is extracted 3 times with an equal volume of n-butanol, and the n-butanol extract is doubled for 200~ 300 mesh silica gel column chromatographic separation, gradient elution with a volume ratio (5:1-1:1) of petroleum ether-acetone mixed solvent, of which the volume ratio of 3:1 petroleum ether-acetone mixed solvent eluted part, After recrystallization, R is CH 3 The yield of the compound of formula I was 5 mg / L, and the fraction elute...
Embodiment 2
[0045] Embodiment 2: The growth inhibition test of the compound of formula I to human acute promyelocytic leukemia HL-60 cell line, human leukemia K562 cell line and mouse lymphocytic leukemia P388 cell line in vitro:
[0046] HL-60, K562 or P388 cells were cultured in RPMI1640 medium containing 10% heat-inactivated fetal bovine serum, 100IU / mL penicillin, 100μg / mL streptomycin and 1mmol / LL-glutamine at 37°C , 5%CO 2 Incubate in a saturated humidity incubator until the cells are 70%-80% confluent. Weigh trypan blue, add a small amount of distilled water to grind, add double distilled water to dilute to 4% storage concentration, filter with filter paper, and store at 4°C. When used, this stock solution was diluted to a working concentration of 0.4% with PBS. Take HL-60, K562 or P388 cells in 3×10 4 Cells / mL density, 1 mL per well was inoculated in a 24-well plate, and then added different concentrations of drugs (final concentrations were 0.1, 0.5, 1, 3, 10, 30, and 100 μM),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com