Expression vector for animal cells including csp-b 5'-sar factor and method for producing recombinant proteins using same
An animal cell and expression vector technology, applied in the field of ld Attachment Reg, can solve the problem of not recording the comparative research content of MAR factor 2 copy and MAR factor 3 copy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Preparation of SAR / MAR Factors
[0072] Preparation of Human CSP-B 5'-SAR and 3'-SAR Factors
[0073] Human natural killer cells (natural killer cells, NK cells and ATCC CRL-2407) genomic DNA. Genomic DNA was prepared from NK cells using a DNA isolation kit (Dneasy Blood & Tissue Kit, Qiagen, Cat. No. 69504), and used as a template for SAR DNA polymerase chain reaction.
[0074] Using the genomic DNA of NK cells as a template, the polymerase chain reaction was performed using primers Cs5S300F (attct tcagc acctc cttaa ttttt ctccc; sequence number 5 in the sequence listing) and primers Cs5S300R (ccagg cagcc aaaga tcagt agttg tgttg; sequence number 6 in the sequence listing) . The conditions of 10 seconds at 98°C, 30 seconds at 60°C, and 2 minutes and 30 seconds at 72°C were repeated 35 times to perform a polymerase chain reaction.
[0075] Next, the polymerase chain reaction product was cloned into pGEM-T vector (Promega, Cat. No. A3600) to prepare pGEMT-CS...
Embodiment 2
[0079] Embodiment 2: Preparation of β-galactosidase expression vector
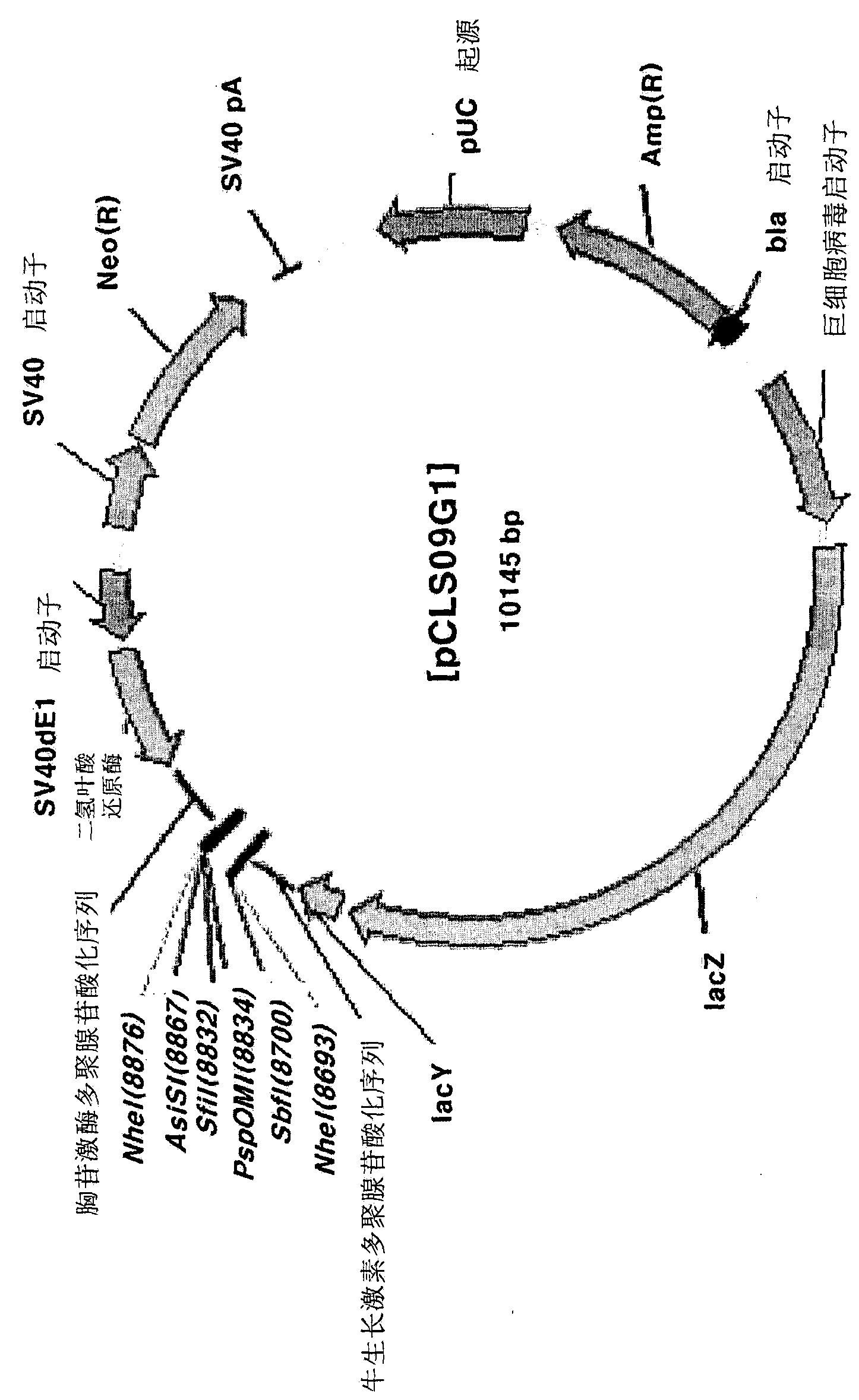
[0080] Preparation of pC06 vector
[0081] In order to prepare DNA containing cytomegalovirus (Cytomegalovirus, CMV) promoter, multiple cloning sites (Multiple Cloning Sites, MCS) and bovine growth hormone (Bovine Growth Hormone, BGH) polyadenylation sequence (polyadenylation sequence, pA) pC06 vector, the following experiments were performed. First, using the pcDNA3.1(-) vector (Invitrogen, Cat.No.V795-20) as a template, use the V6-F primer (aagct tggat ccgaa ttcat cgatg gccgg ccggt accct cgagc tgtgc cttct agttg ccagc; sequence 13 ) and V6-R primers (gctag ctaga gcccc agctg gttct ttccg; sequence No. 14 in the sequence listing) performed polymerase chain reaction. The conditions of 98° C. for 10 seconds, 60° C. for 30 seconds, and 72° C. for 1 minute were repeated 30 times to perform a polymerase chain reaction. Then, the polymerase chain reaction product was cloned into a pcDNA3.3-TOPO vector (Invitrog...
Embodiment 3
[0094] Embodiment 3: Preparation of the β-galactosidase expression vector comprising SAR / MAR
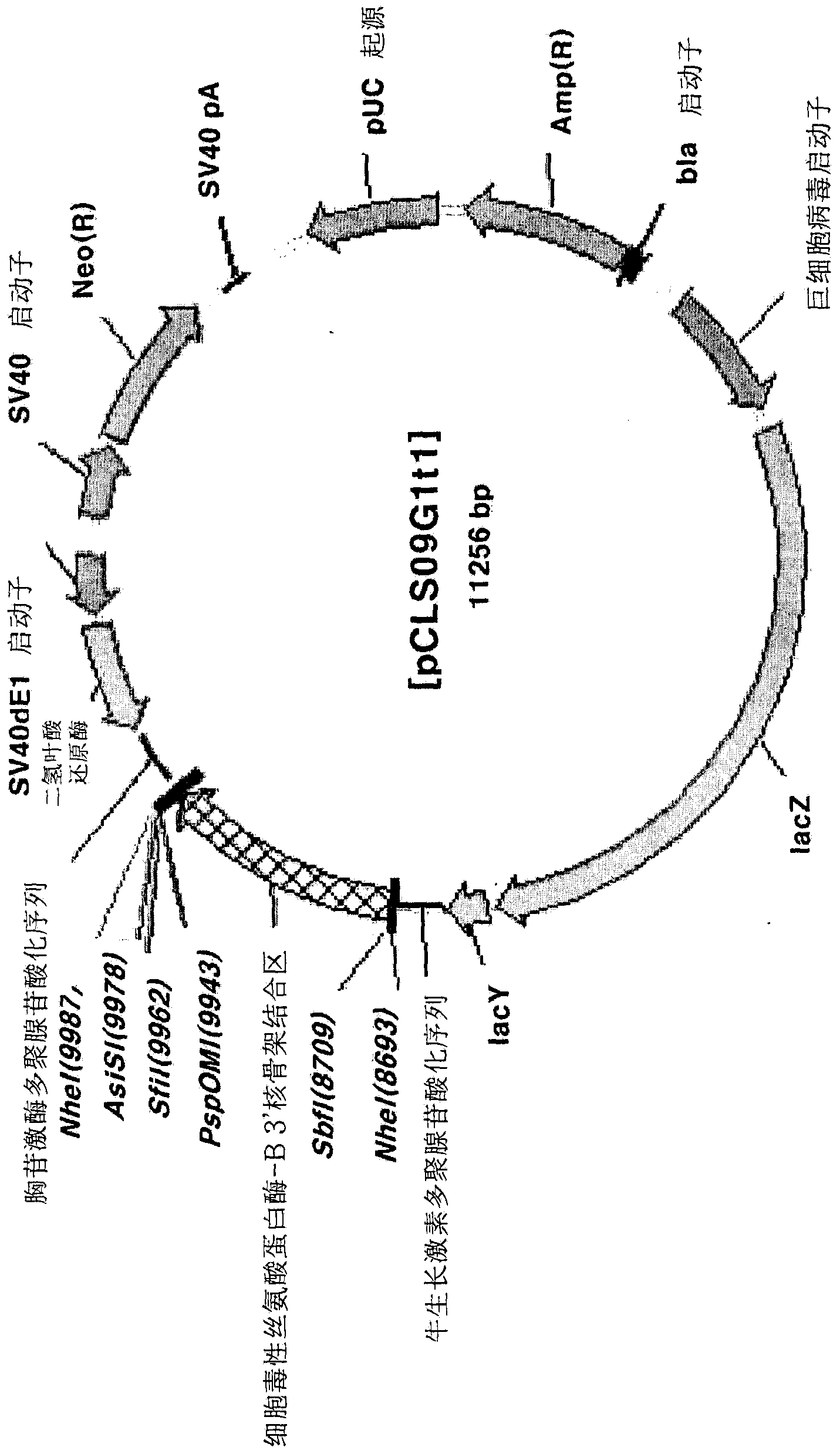
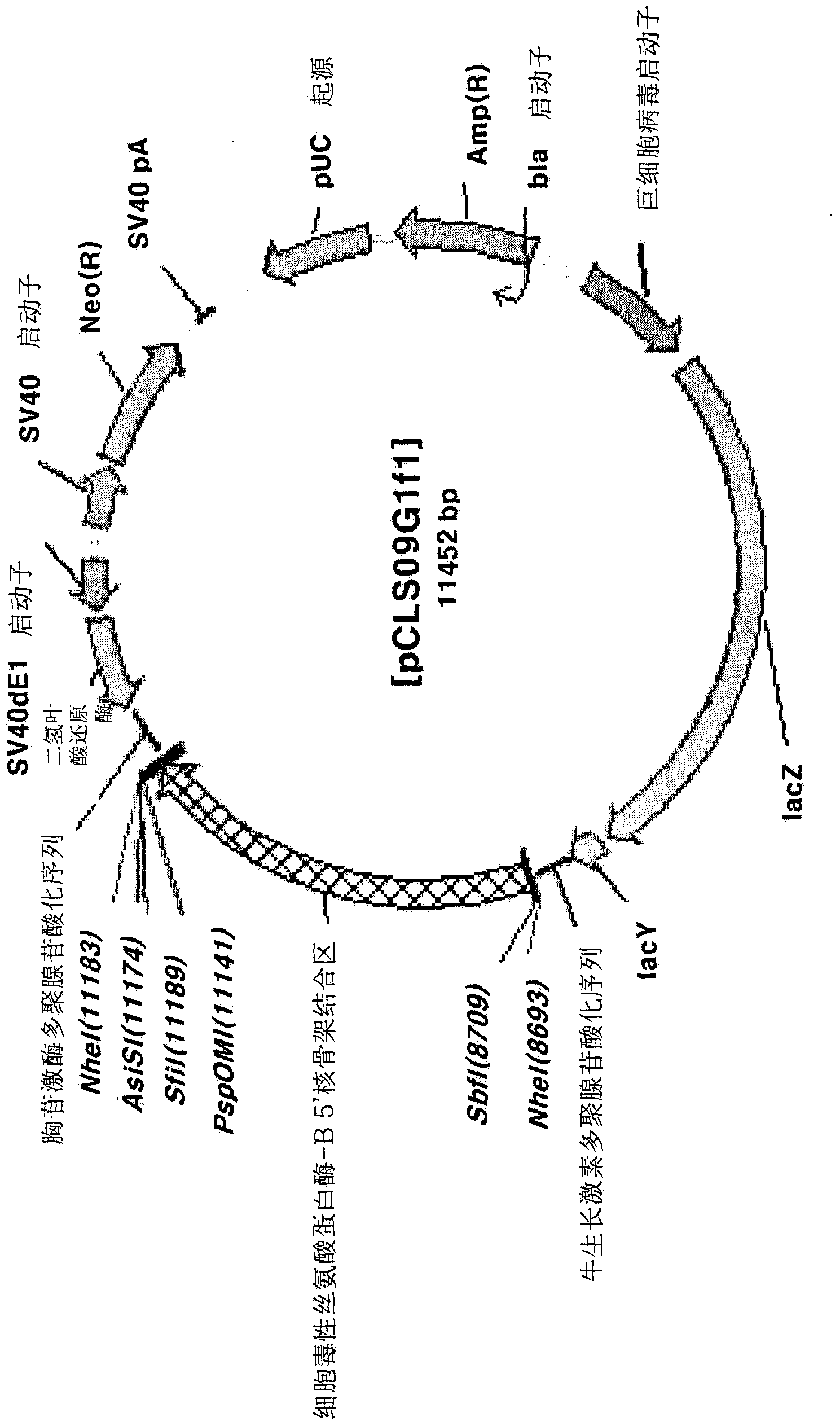
[0095]Preparation of pCLS09G1t1 vector
[0096] In order to insert the CSP-B3'-SAR factor between the SbfI and PspOMI restriction endonuclease sites of the pCLS09G1 vector to prepare the pCLS09G1t1 vector, the following experiment was performed. First, using the pGEMT-CS3S.1.2.k vector as a template, use the cs3sSbfIF primer (aattc ctgca gggga tccca ttctc cttga tgtac taat; sequence number 26 in the sequence listing) and cs3sPsp1R primer (aattg ggccc gaatt caaac aactc aatag caaga aac; sequence listing 27 sequence) to perform polymerase chain reaction, thereby amplifying the CSP-B3'-SAR factor. The conditions of 10 seconds at 98°C, 30 seconds at 60°C, and 2 minutes and 30 seconds at 72°C were repeated 30 times to perform a polymerase chain reaction. Then, the polymerase chain reaction product was cloned into the Sbf I and PspOM I restriction endonuclease sites of the pCLS09G1 vector ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com