Corneal contact lens drug carrier with bionic characteristics, and preparation method thereof
A technology of contact lenses and characteristics, applied in medical science, surgery, etc., can solve the problems of limited drug loading and controlled release capabilities, and achieve the effect of improving hydrophilic performance, large social and economic benefits, and increased comfort
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
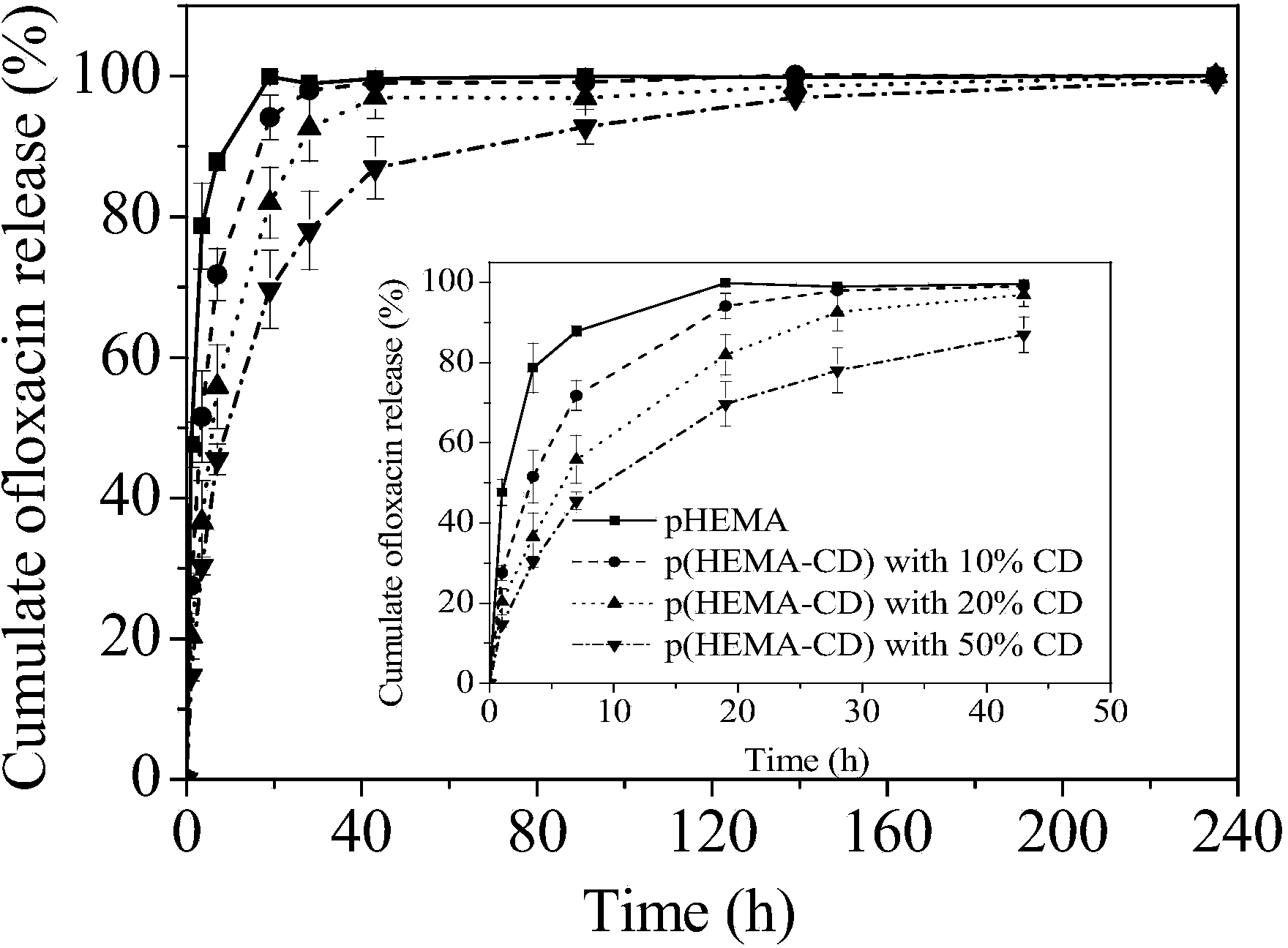
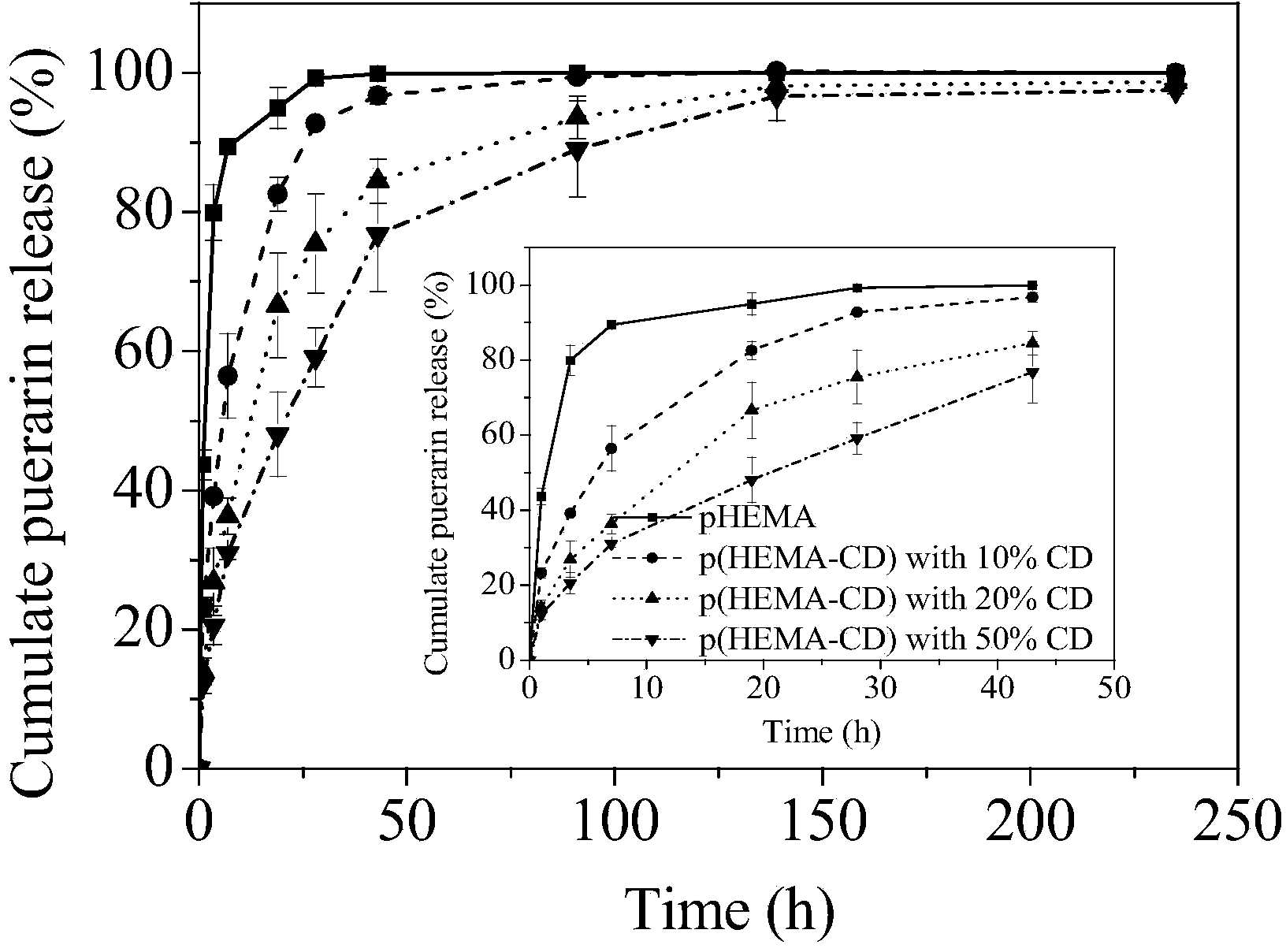
[0050] Add hydroxyethyl methacrylate (HEMA), cyclodextrin derivatives (the functionality of the double bond is 1.3) monomers into the container at a mass ratio of 1:1, and then add 1wt% MPC aqueous solution, wherein the mass of MPC 0.5% of the total mass of HEMA and cyclodextrin (M MPC :M HEMA+CD ); then add ammonium persulfate (APS), tetramethylethylenediamine (TMEDA) redox initiator with a molar ratio of 1:1, wherein the quality of the initiator is 0.5% of the total mass of HEMA and cyclodextrin derivatives (M APS+TMEDA :M HEMA+CD ); the whole system adds or maintains the mass content of solvent water to 35% (M Water :M 总体系 ). Stir the reaction system evenly and pour it into a mold, react at 60° C. for 1 hour, then soak in water for demoulding to obtain a terpolymer hydrogel. The equilibrium water content of the hydrogel is between 52% and 60%, the amount of adsorbed protein (BSA) is 6.8±0.3mg / g hydrogel, and the amount of drug loaded is 27±3.1mg / g. The drug release cu...
Embodiment 2
[0052] Add hydroxyethyl methacrylate (HEMA), cyclodextrin derivatives (the functionality of the double bond is 1.3) monomers into the container at a mass ratio of 4:1, and then add 1wt% MPC aqueous solution, wherein the mass of MPC 0.5% of the total mass of HEMA and cyclodextrin (M MPC :M HEMA+CD ); then add ammonium persulfate (APS), tetramethylethylenediamine (TMEDA) redox initiator with a molar ratio of 1:1, wherein the quality of the initiator is 0.5% of the total mass of HEMA and cyclodextrin derivatives (M APS+TMEDA :M HEMA+CD ); the whole system adds or maintains the mass content of solvent water to 35% (M Water :M 总体系 ). Stir the reaction system evenly and pour it into a mold, react at 60° C. for 1 hour, then soak in water for demoulding to obtain a terpolymer hydrogel. The equilibrium water content of the hydrogel is between 52% and 60%, the amount of adsorbed protein (BSA) is 7.0±0.2mg / g hydrogel, and the amount of drug loaded is 22±1.7mg / g. The drug release cu...
Embodiment 3
[0054] Add hydroxyethyl methacrylate (HEMA), cyclodextrin derivatives (the functionality of the double bond is 1.3) monomers into the container at a mass ratio of 9:1, and then add 1wt% MPC aqueous solution, wherein the mass of MPC 0.5% of the total mass of HEMA and cyclodextrin (M MPC :M HEMA+CD ); then add ammonium persulfate (APS), tetramethylethylenediamine (TMEDA) redox initiator with a molar ratio of 1:1, wherein the quality of the initiator is 0.5% of the total mass of HEMA and cyclodextrin derivatives (M APS+TMEDA :M HEMA+CD ); the whole system adds or maintains the mass content of solvent water to 35% (M Water :M 总体系 ). Stir the reaction system evenly and pour it into a mold, react at 60° C. for 1 hour, then soak in water for demoulding to obtain a terpolymer hydrogel. The equilibrium water content of the hydrogel is between 52% and 60%, the amount of adsorbed protein (BSA) is 7.1±0.3mg / g hydrogel, and the amount of drug loaded is 18±1.5mg / g. The drug release cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com