Dendritic polyketal as well as preparation method and application thereof
A dendritic and polyketal technology, which is applied in the field of preparation of dendritic polyketals and derivatives, can solve problems such as blank application research and no relevant reports, and achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
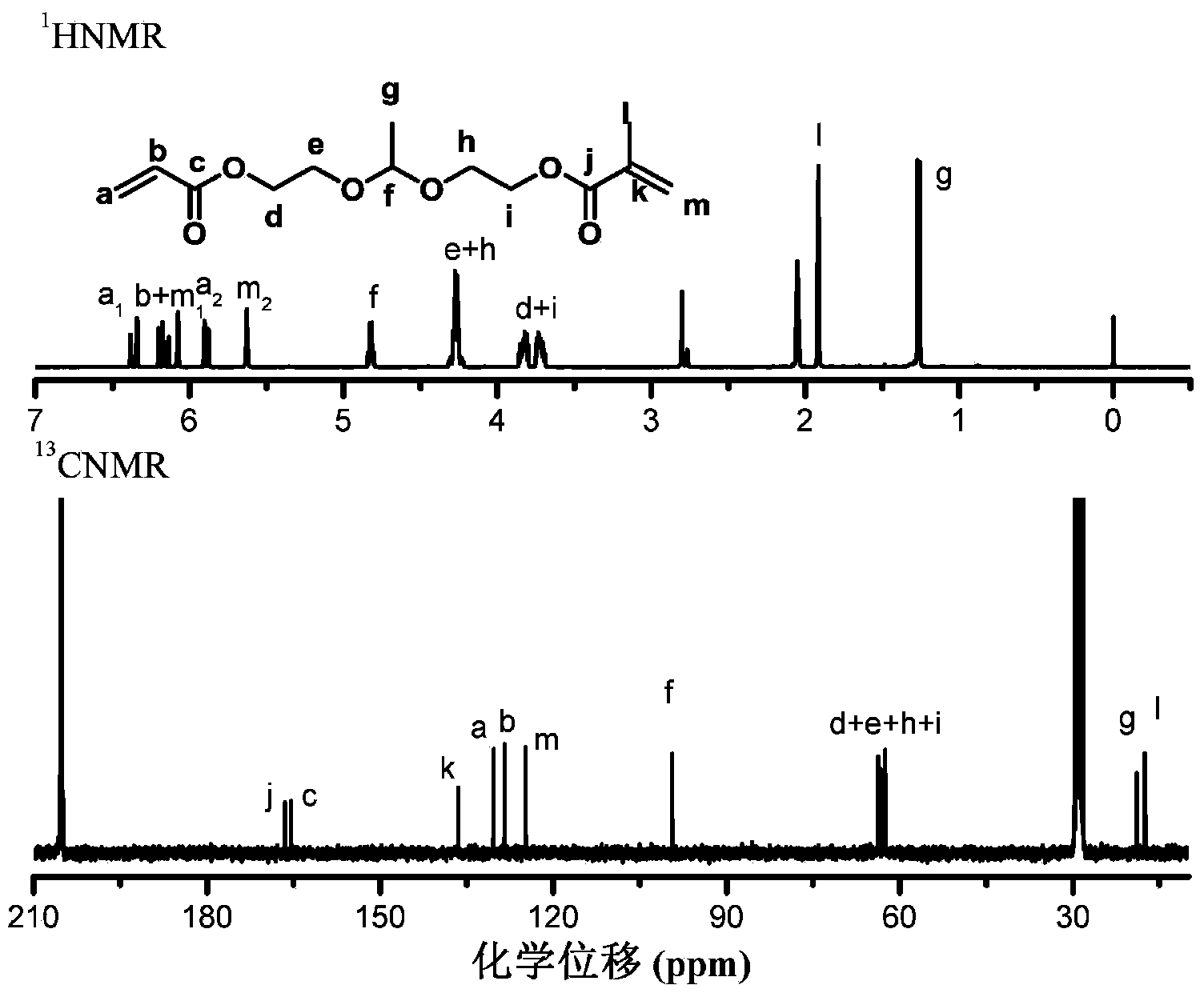
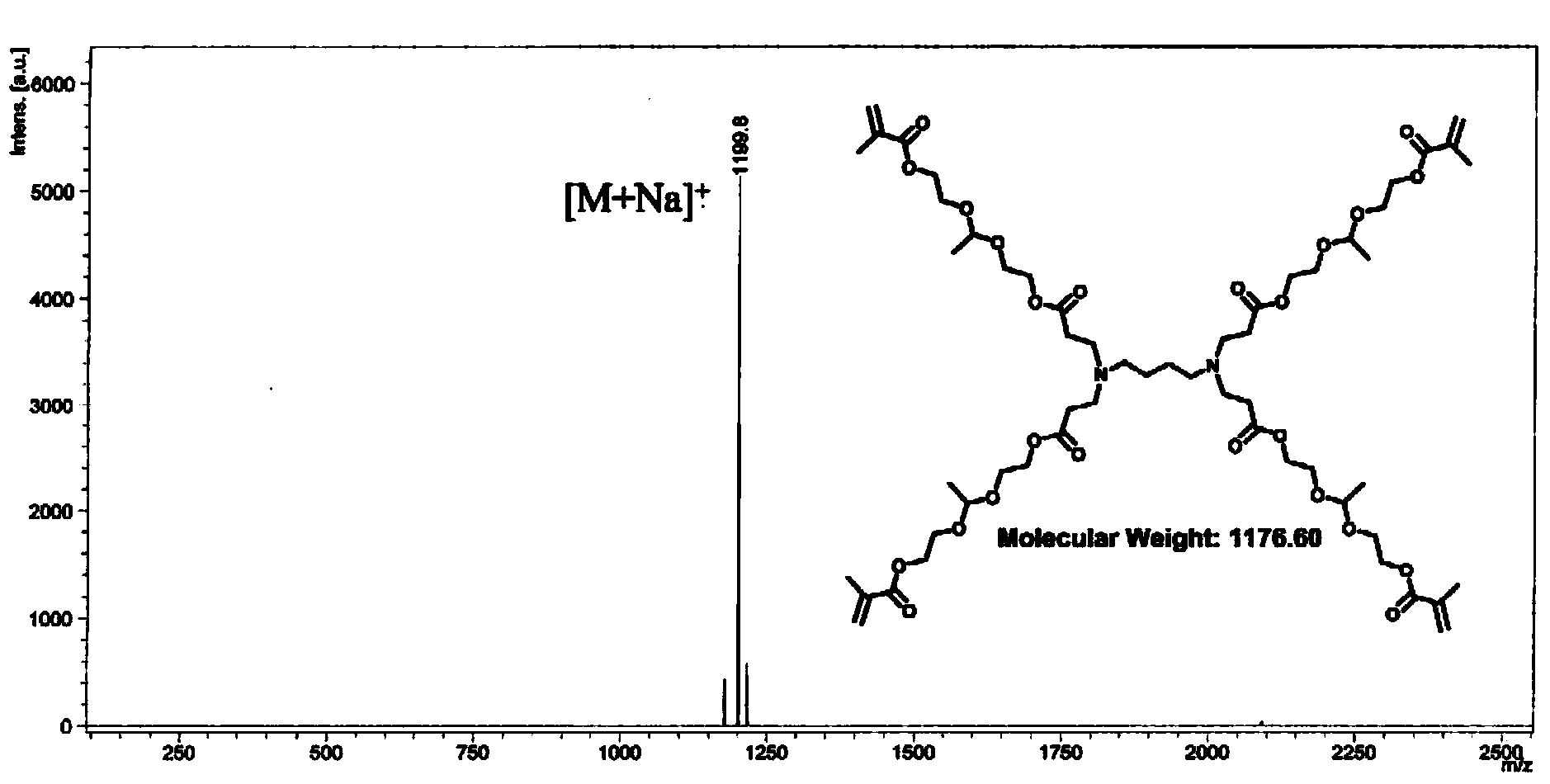
[0109] Preparation of dendritic polyketal G1-[ene] with algebra G=1 4 (The core (C) in the general structural formula I of the aforementioned dendritic polyketal is butanediamine; the terminal group TF is an alkenyl group; D in the general structural formula II of the branch unit is a nitrogen atom, A and B is an alkylene group containing an ester bond, X and Y are a hydrogen atom and a methyl group, respectively).
[0110] 1) Preparation of monomers containing ketal groups (acrylate)-ketal-(methacrylate)
[0111] Dissolve hydroxyethyl acrylate, hydroxyethyl methacrylate, and acetaldehyde in tetrahydrofuran at a molar ratio of 1:1:1, add 0.1 equivalent of pyridinium p-toluenesulfonate (PPTS) and an appropriate amount of 5A molecular sieve, at room temperature After reacting for 3 hours, potassium carbonate was added to terminate the reaction, and the filtrate was retained after filtration to obtain the crude product, and then the crude product was passed through silica gel co...
Embodiment 2
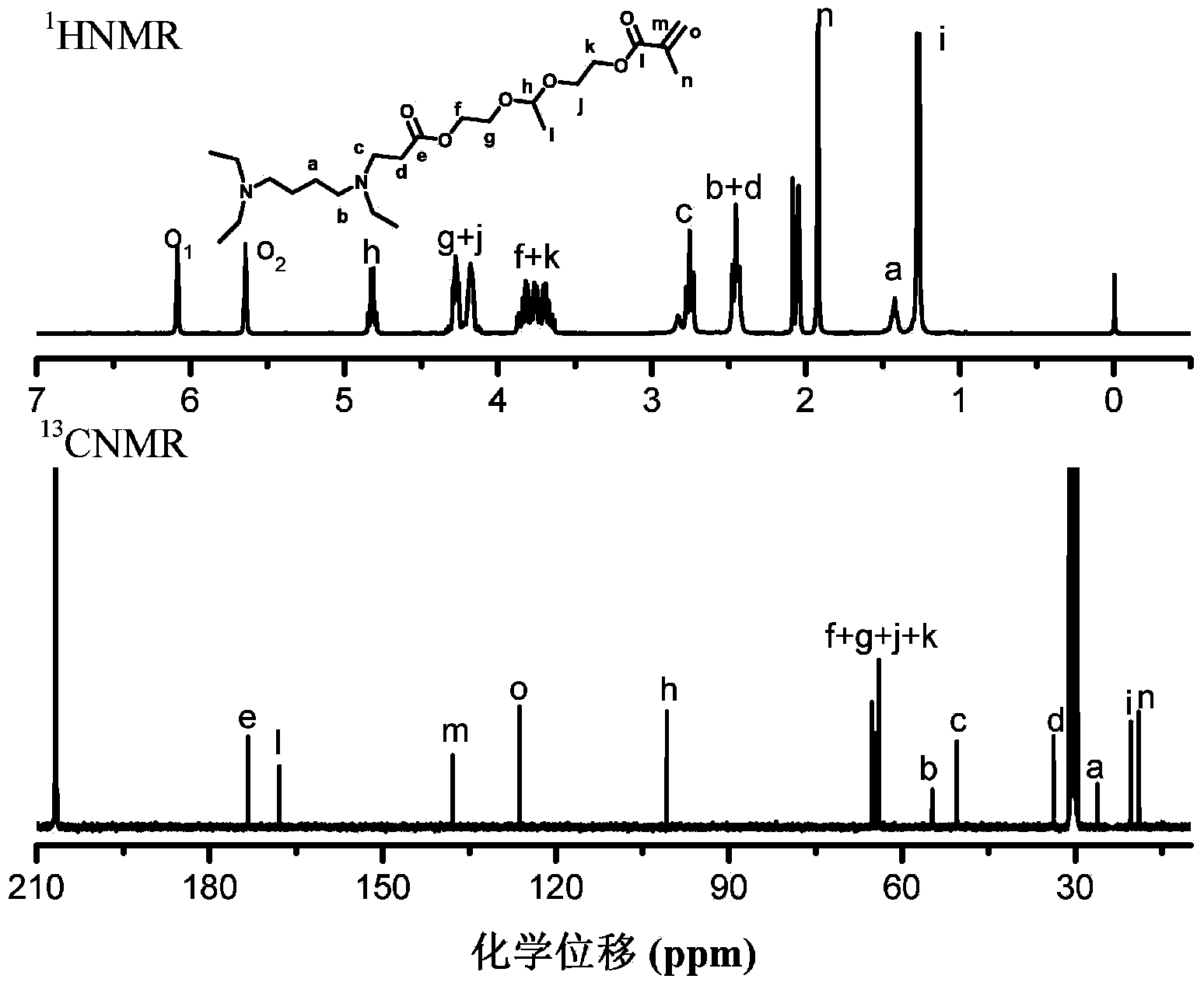
[0117] Preparation of dendritic polyketal G1'-[NH with algebra G=1' 2 ] 4 (The core (C) in the general structural formula I of the aforementioned dendritic polyketal is butanediamine; the terminal group TF is an amine group; D in the general structural formula II of the branch unit is a nitrogen atom, A and B is an alkylene group containing an ester bond, X and Y are a hydrogen atom and a methyl group, respectively).
[0118] The dendritic polyketal G1-[ene] of the algebra G=1 that step (2) obtains in embodiment 1 4 Dissolve cysteamine and cysteamine in dimethyl sulfoxide at a molar ratio of 1:6, and react for 30 minutes at room temperature; after the reaction, add 10 times the volume of dimethyl sulfoxide to dichloromethane for dilution, and then wash with water three times , the organic phase was dehydrated and vacuum dried to obtain a light yellow viscous liquid, which is the dendritic polyketal G1'-[NH 2 ] 4 .
Embodiment 3
[0120] Preparation of dendritic polyketal G2-[ene] with algebra G=2 8 (The core (C) in the general structural formula I of the aforementioned dendritic polyketal is butanediamine; the terminal group TF is an alkenyl group; D in the general structural formula II of the branch unit is a nitrogen atom, A and B is an alkylene group containing an ester bond, X and Y are a hydrogen atom and a methyl group, respectively).
[0121] Repeat the reaction of step (2) in Example 1, only change the charge ratio wherein into the dendritic polyketal G1 '-[NH 2 ] 4 The molar ratio of the ketal group-containing monomer (acrylate)-ketal-(methacrylate) obtained in step (1) in Example 1 is 1:16, and the dendritic polymer with algebra G=2 can be obtained. Ketal G2-[ene] 8 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com