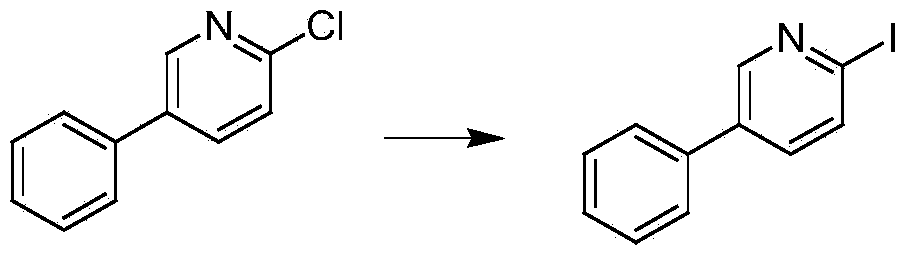
Method for preparing 2-iodine-5-phenylpyridine
A technology of phenylpyridine and iodonium salt, applied in the industrial production field of 2-iodo-5-phenylpyridine, can solve problems such as high cost and unsuitability for production, and achieve the effects of improving income, saving production cost and stabilizing process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A 10-liter glass reactor is equipped with mechanical stirring and a thermometer. Under the protection of nitrogen, add 500 g of raw material 2-chloro-5-phenylpyridine, a certain amount of cuprous iodide and 1,10-phenanthroline, relative to the raw material 2-chloro -5-Phenylpyridine 1.1 molar equivalents of sodium iodide, 3 liters of dimethyl sulfoxide as a solvent, heated to 160 ° C for reaction, stop the reaction when there is no raw material 2-chloro-5-phenylpyridine remaining in the system, drop To room temperature, distill under reduced pressure to recover the solvent dimethyl sulfoxide, so that it can be used mechanically next time, add 2 liters of tetrahydrofuran to the residue in the reactor, stir for half an hour, filter with suction, and concentrate the filtrate to dryness to obtain the product 2-iodo-5- For the crude product of phenylpyridine, a certain amount of methanol was added to the crude product for recrystallization to obtain the pure product with a mo...
Embodiment 2
[0025] A 10-liter glass reactor is equipped with a mechanical stirrer and a thermometer. Under the protection of nitrogen, add 500 g of the raw material 2-chloro-5-phenylpyridine, a certain amount of cuprous iodide and 1,2-cyclohexanediamine, relative to the raw material 2- Chloro-5-phenylpyridine 1.1 molar equivalent of potassium iodide, 3 liters of N,N-dimethylformamide as a solvent, heated to 150°C to react, stop when there is no raw material 2-chloro-5-phenylpyridine remaining in the system Reaction, lowered to room temperature, and the solvent N,N-dimethylformamide was recovered by distillation under reduced pressure, so as to be used mechanically next time, 2 liters of ethyl acetate was added to the residue in the reactor, stirred for half an hour, filtered with suction, and the filtrate was concentrated to dryness , to obtain the crude product of 2-iodo-5-phenylpyridine, a certain amount of ethanol was added to the crude product, and recrystallized to obtain the pure pro...
Embodiment 3
[0027] A 10-liter glass reactor is equipped with a mechanical stirrer and a thermometer. Under the protection of nitrogen, add 500 g of raw materials 2-chloro-5-phenylpyridine, a certain amount of cuprous iodide and N,N'-dimethyl-1,2-cyclo Hexamethylenediamine, 1.1 molar equivalents of potassium iodide relative to the raw material 2-chloro-5-phenylpyridine, 3 liters of N-methylpyrrolidone as a solvent, heated to 150°C for reaction, when there is no raw material 2-chloro-5-benzene in the system Stop the reaction when base pyridine remains, drop to room temperature, and recover the solvent N-methylpyrrolidone by distillation under reduced pressure, so that it can be used mechanically next time, add 2 liters of 1,2-dichloroethane to the residue in the reactor, stir for half an hour, After suction filtration, the filtrate was concentrated to dryness to obtain the crude product 2-iodo-5-phenylpyridine. A certain amount of ethanol was added to the crude product for recrystallization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com