Cyclopentadiene-bridging dimeric perylene diimide compound and preparation method thereof
A bridging technology of bisperylene diimide and cyclopentadiene, which is applied in the fields of chemical instruments and methods, organic chemistry, organic dyes, etc., can solve the problems of complicated reaction steps, strict reaction conditions, and low reaction yield, etc. Achieve the effect of short reaction time, simple method and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
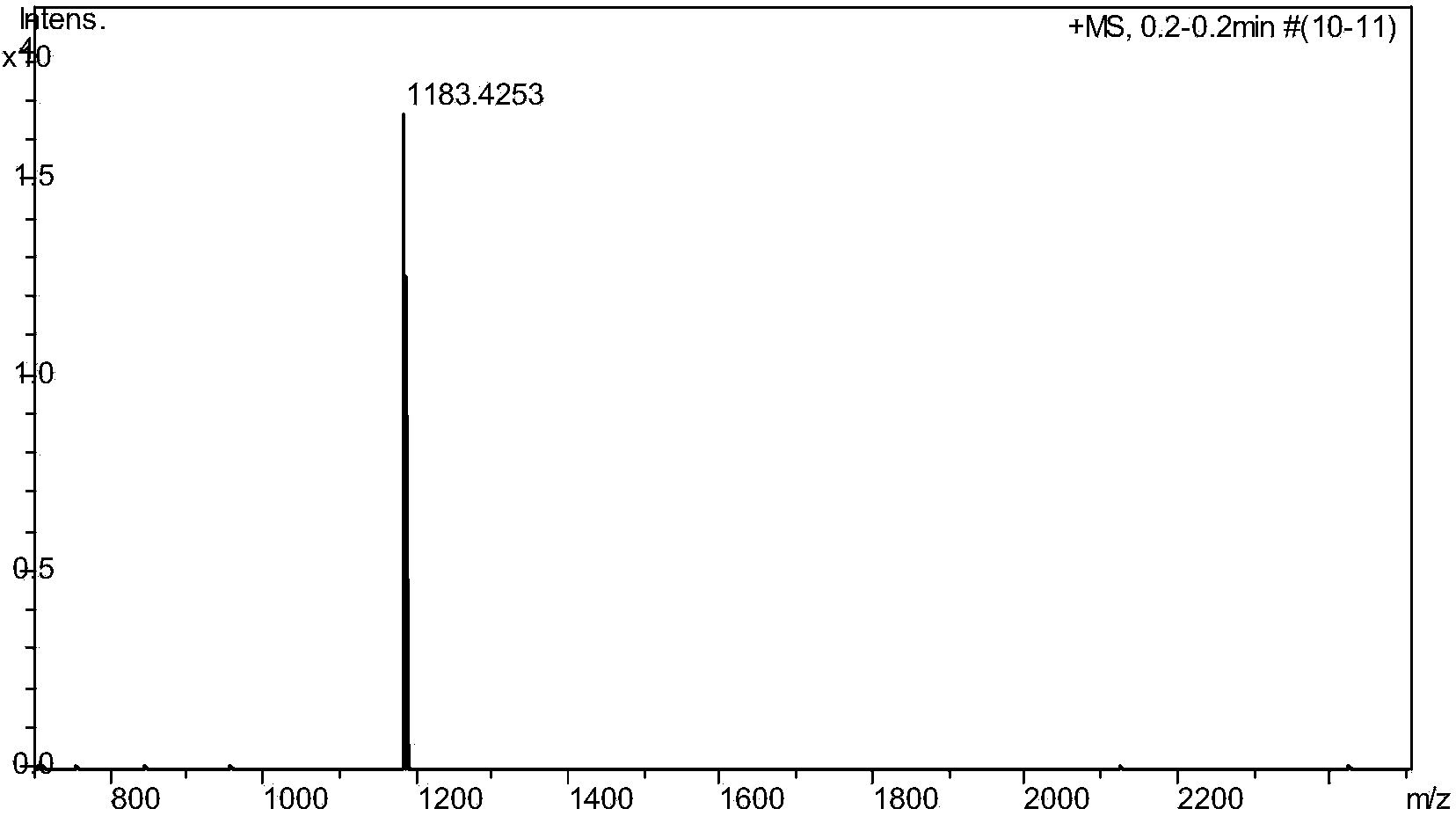
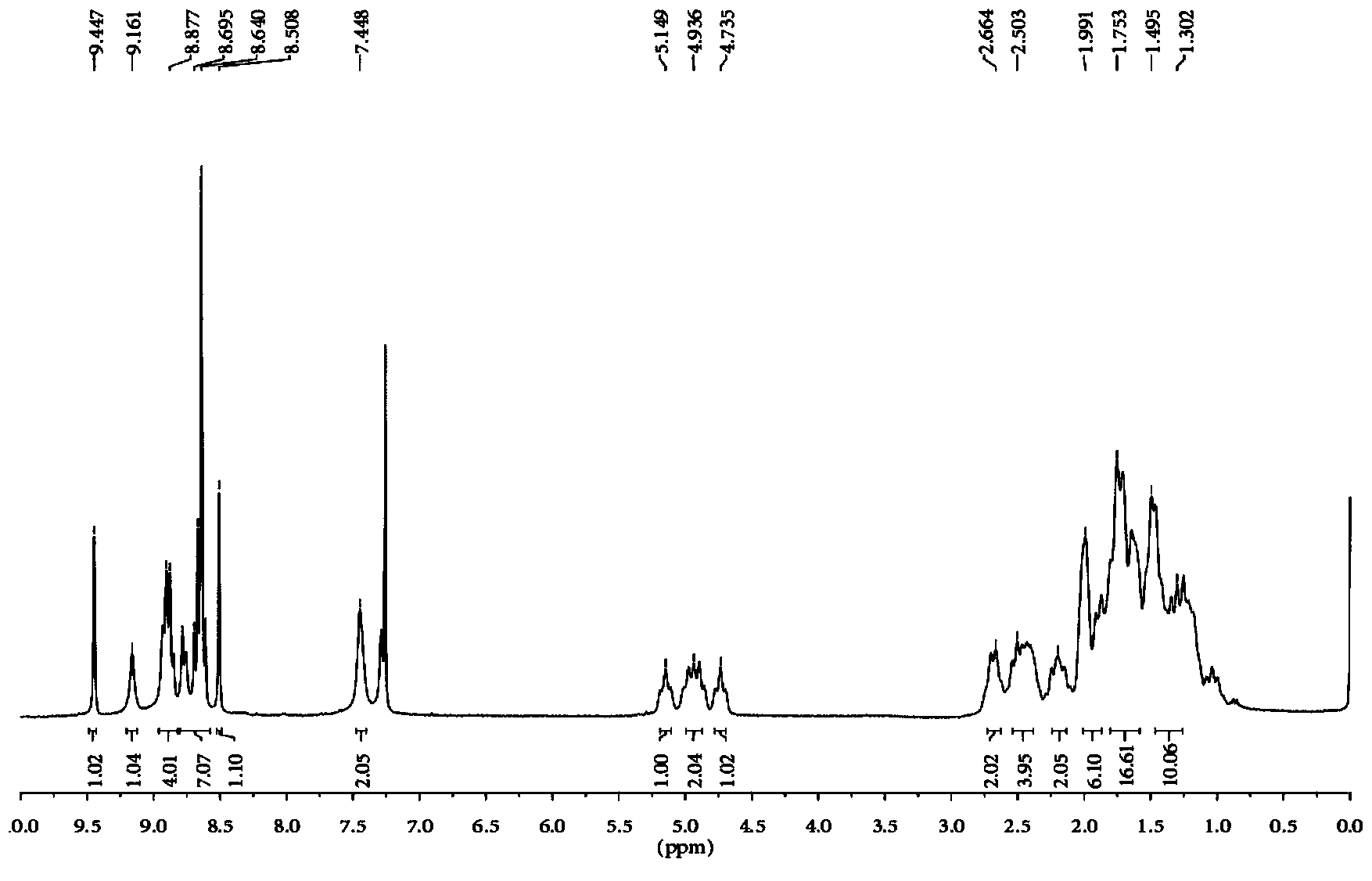
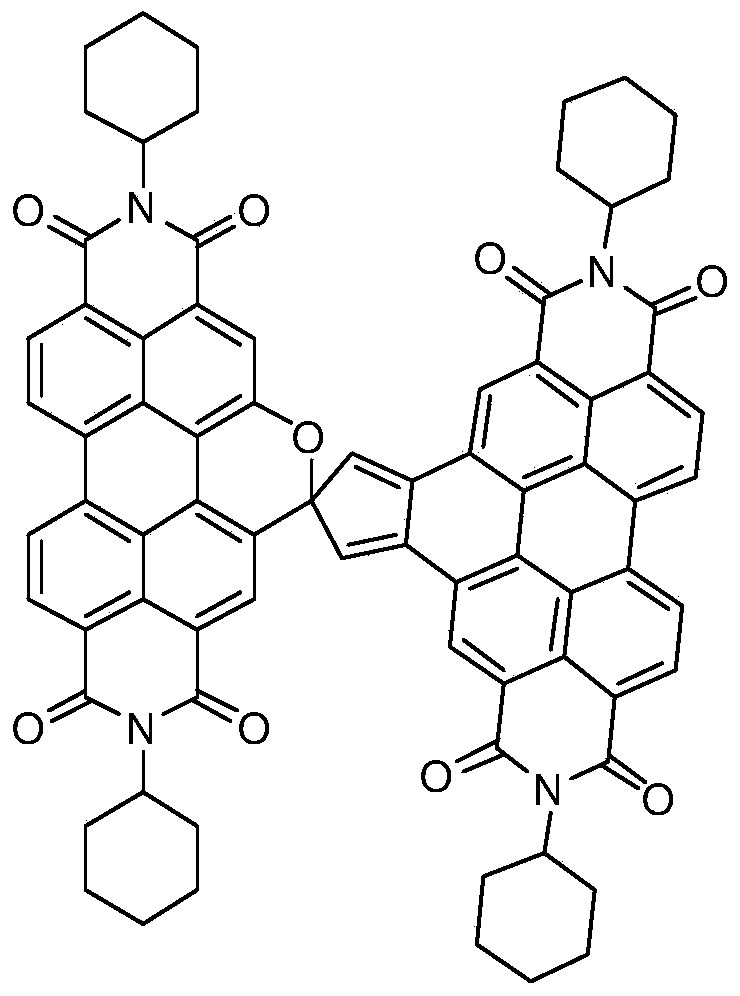
[0025] Take 600 mg of N,N'-dicyclohexyl-1-nitro-3,4:9,10-perylenetetracarboxylic diimide, 0.30 mL of cyclopentadiene and 415 mg of potassium carbonate dissolved in 20 mL of N -Methylpyrrolidone, stirred at room temperature, reacted for 2 hours, the reaction solution was dropped into 100 ml of 2mol / L dilute hydrochloric acid, precipitated, filtered with suction, washed 3 times with water, and dried. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane:ethyl acetate=20:1. The product was obtained in 408 mg, yield 69%. Such as figure 1 , figure 2 As shown, by mass spectrometry and proton nuclear magnetic spectrum analysis of the product, it is determined that the perylene derivatives structural formula prepared by this method is as follows image 3 shown.
[0026]
Embodiment 2
[0028] Take 600 mg of N,N'-dicyclohexyl-1-nitro-3,4:9,10-perylenetetracarboxylic diimide, 0.30 mL of cyclopentadiene and 600 mg of potassium bicarbonate dissolved in 20 mL In the mixed solution of N,N'-dimethylacetamide and tetrahydrofuran (VN,N'-dimethylacetamide: V tetrahydrofuran = 1:1), stir at room temperature, react for 3 hours, and drop the reaction liquid into 100 ml In 2mol / L dilute hydrochloric acid, a precipitate precipitated, filtered with suction, washed with water three times, and dried. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane:ethyl acetate=20:1. The product was obtained in 198 mg, yield 34%.
[0029]
Embodiment 3
[0031] Take 600 mg of N,N'-dicyclohexyl-1-nitro-3,4:9,10-perylenetetracarboxylic diimide, 0.30 mL of cyclopentadiene and 380 mg of sodium carbonate dissolved in 20 mL of N , N'-dimethylformamide, stirred and reacted at 50°C for 0.5 hours, the reaction liquid was dropped into 100 ml of 2mol / L dilute hydrochloric acid, precipitated, filtered with suction, washed 3 times with water, and dried. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane:ethyl acetate=20:1. The product was obtained in 278 mg, yield 47%.
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com