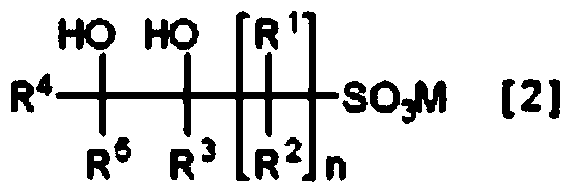
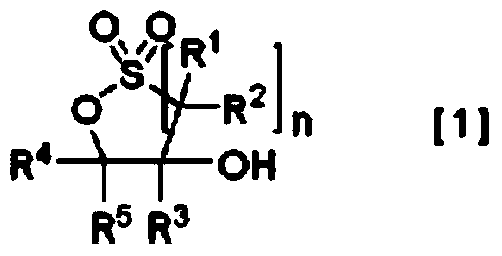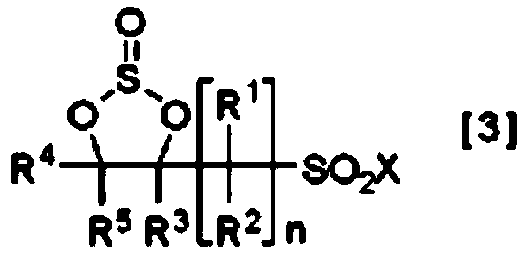Method for producing cyclic sulfonic acid ester and intermediate thereof
A technology of acid anhydrides and compounds, applied in the field of manufacture of cyclic sulfonic acid esters (sultones), can solve problems such as low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 12
[0090] Synthesis example 12, Synthesis of 3-dihydroxypropanesulfonate sodium (reaction formula [I])
[0091] To a solution obtained by dissolving 120 g of sodium sulfite (924 mmol, content: 97.0%; manufactured by Wako Pure Chemical Industries, Ltd.) in 400 ml of water, 107.2 g of 3-chloro-1,2-propanediol (970 mmol; manufactured by Wako Pure Chemical Industries, Ltd. fabrication), heated to reflux for 1 h. After the reaction is terminated, concentrate the reaction solution, then add 750 ml of methanol to the concentrated residue, filter the resulting crystals, and dry the obtained crystals to obtain white crystals that are 2,3-dihydroxy Sodium propanesulfonate 202.6g (content: 67.2%, yield: 82.7%). Furthermore, the content of 2,3-dihydroxypropanesulfonate sodium, by adopting 1 Calculated by the internal standard method of H-NMR. In addition, it was confirmed that sodium chloride crystals were mixed as by-products in the above-mentioned white crystals. Shown below 1 Measure...
Embodiment 11
[0094] Example 11, Synthesis of 3,2-dioxotetrahydrothiophene-2-oxo-4-yl-methanesulfonyl chloride (the first step; reaction formula [II])
[0095] Make 80.0g (301mmol, content: 67.2%) in the total amount of 2,3-dihydroxypropanesulfonate obtained in Synthesis Example 1 in N, N-dimethylformamide (DMF) 110.3g (1509mmol; Wako Pure Pharmaceutical Co., Ltd.), and cooled to -20°C to 10°C. 107.7 g (905 mmol; manufactured by Wako Pure Chemical Industries, Ltd.) of thionyl chloride was added dropwise to the cooled suspension, followed by stirring at room temperature for 3 hours to allow a reaction. After the reaction was terminated, the reaction solution was added to 400 ml of water cooled to -20°C to 10°C, then 200 ml of toluene was added to the mixed solution, stirred, and the organic layer was separated. After the separated organic layer was washed with water, the organic layer was concentrated to obtain 1,3,2-dioxotetrahydrothiophene-2-oxyethylene-4-yl-methanol of the above-mentione...
Embodiment 22
[0101] Example 2 Synthesis of 2-hydroxy-1,3-propanesultone (second step; reaction formula [III])
[0102]To 4.21 g (19.1 mmol) of the total amount of 1,3,2-dioxotetrahydrothiophene-2-oxo-4-yl-methanesulfonyl chloride obtained in Example 1, under ice cooling, add 12N hydrochloric acid 1.75g (hydrogen chloride; 21.0mmol, water; 62.2mmol; manufactured by Wako Pure Chemical Industries, Ltd.), stirred at room temperature for 30 minutes, and allowed to react. After the reaction was terminated, 50 ml of ethyl acetate was added to the reaction liquid, followed by stirring, and then, the organic layer was separated. After the separated organic layer was washed with water, the organic layer was concentrated, then, toluene was added to the concentrated residue, and the resulting crystal was collected by filtration, and the obtained crystal was dried to obtain a white crystal, which is the 2- Hydroxy-1,3-propane sultone 2.51 g (yield: 95%). Shown below 1 Measurement results of H-NMR....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com