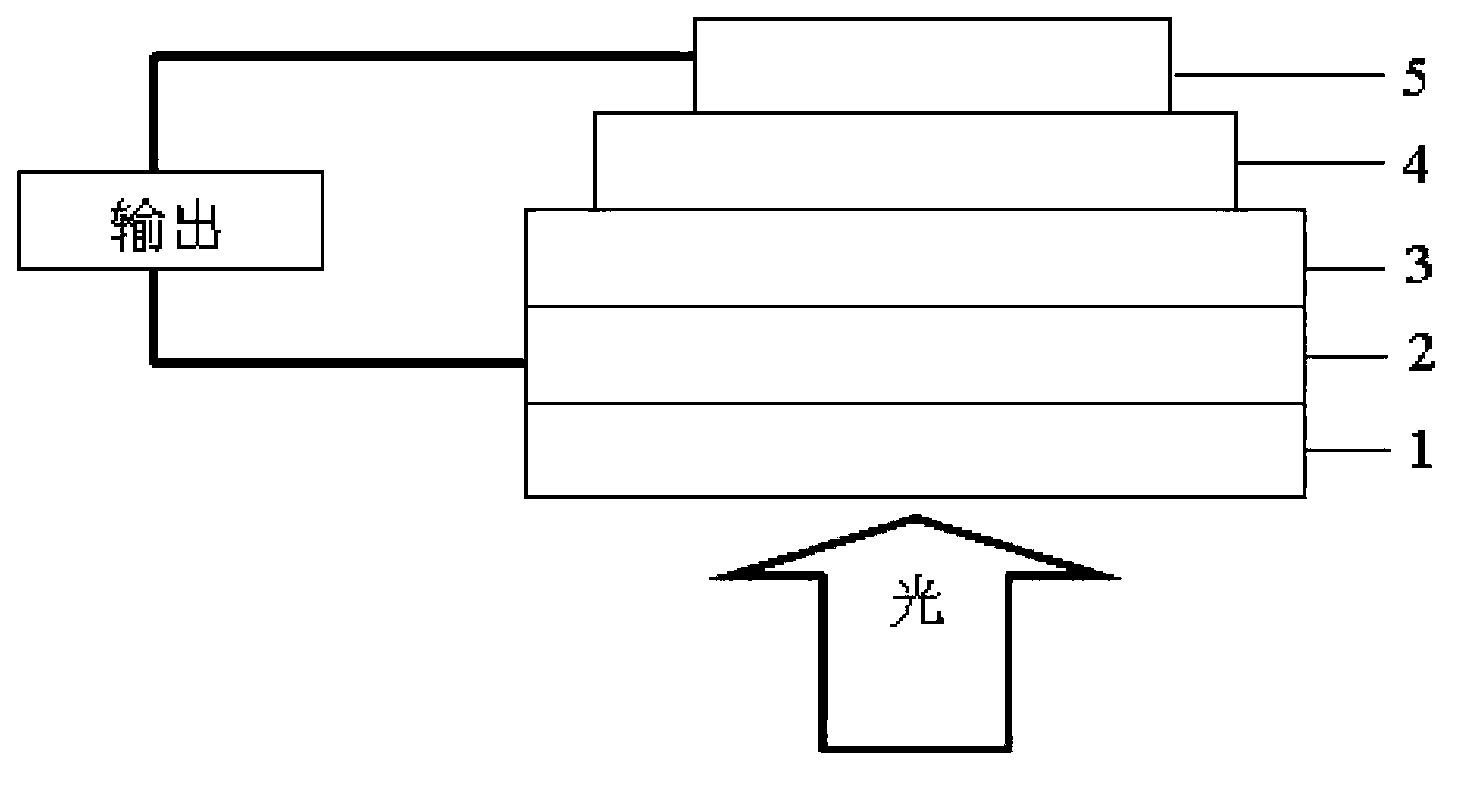
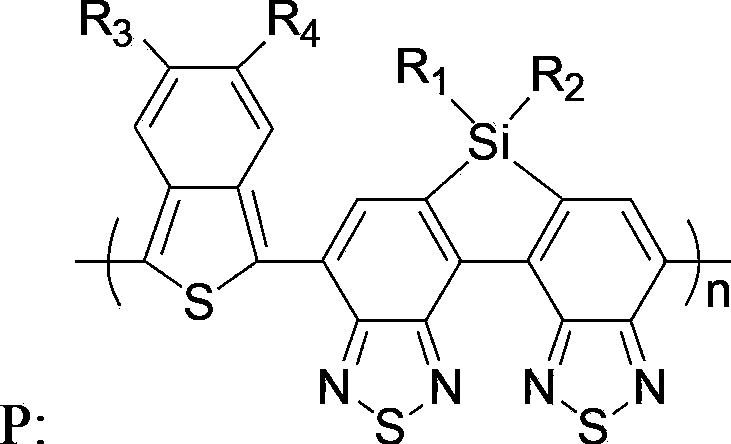
Benzothiophene-silole-di(diazosulfide)-containing copolymer as well as preparation and application thereof
A technology of benzothiadiazole and benzothiophene, which is applied in the field of benzothiophene-thiarolodipolymers and its preparation, can solve the problems of low energy conversion efficiency and achieve narrow band gap, strong conjugation properties, Good planar structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] A benzothiophene-thiarolobis(benzothiadiazole) copolymer, namely poly{4,5-dioctylbenzothiophene-6,6 dioctylthiarolo[3,2-e :4,5-e]bis(benzothiadiazole)} (n=52), denoted as copolymer P1, the structural formula is as follows:
[0067]
[0068] The preparation method comprises the following steps:
[0069] (1), the preparation method of 4,9-dibromo-6,7-dioctyl-benzo[2,1-e:3,4-e]bis(benzothiadiazole) (A1) includes the following Steps:
[0070]
[0071] (1) Preparation of compound 5-nitro-2,1,3 benzothiadiazole, the reaction formula is:
[0072]
[0073] Add 4-nitrobenzene-1,2-diamine (22.95g, 0.15mol) and 100mL thionyl chloride into a three-necked flask, stir and slowly add 2mL pyridine dropwise, heat and then reflux at 80~90°C for 24h , stop the reaction, heat to 80°C and spin evaporate excess thionyl chloride, then cool the reaction product to room temperature, pour it into a large amount of water, stir, filter, wash with water and then vacuum dry to obtain the...
Embodiment 2
[0099] A benzothiophene-thiarolobis(benzothiadiazole) copolymer, that is, poly{4,5-bis(n-eicosyl)benzothiophene-6,6 dimethylthiadiazole[ 3,2-e:4,5-e] bis(benzothiadiazole)} (n=25), denoted as copolymer P2, the structural formula is as follows:
[0100]
[0101] The preparation method comprises the following steps:
[0102] (1), the preparation method of 6,6-dimethyl-4,8-dibromothiarolo[3,2-e:4,5-e]bis(benzothiadiazole) (A2) includes the following Steps:
[0103] 4,4'-dibromo-6,6'-diiodo-bi-2,1,3-benzothiadiazole was prepared according to steps (1)-(4) of step (1) of Example 1.
[0104] Compound C2, ie, dimethyldichlorosilane, having the structural formula shown in formula C2 is provided.
[0105] Add 4,4'-dibromo-6,6'-diiodo-bi-2,1,3 benzothiadiazole (3.4g, 5mmol) and 50mL DMF into a three-necked flask, blow nitrogen into it and stir for 20min, slowly Add 4.0mL of n-butyllithium n-hexane solution (2.5M, 0.01mol) dropwise, drop it over in 0.5 hours, control the temperatu...
Embodiment 3
[0120] A benzothiophene-thiarolobis(benzothiadiazole) copolymer, that is, poly{benzothiophene-6,6 bis(n-eicosyl)thiarolo[3,2-e:4 ,5-e] bis(benzothiadiazole)} (n=24), denoted as copolymer P3, the structural formula is as follows:
[0121]
[0122] The preparation method comprises the following steps:
[0123] (1), 6,6-bis(n-eicosyl)-4,8-dibromothiarolo[3,2-e:4,5-e]bis(benzothiadiazole) (A3) The preparation method comprises the following steps:
[0124]4,4'-dibromo-6,6'-diiodo-bi-2,1,3-benzothiadiazole was prepared according to steps (1)-(4) of step (1) of Example 1.
[0125] A compound C3 having a structural formula as shown in the formula C3 is provided, that is, bis(n-eicosyl)dichlorosilane.
[0126] Add 4,4'-dibromo-6,6'-diiodo-bi-2,1,3 benzothiadiazole (3.4g, 5mmol) and 50mL DMF into a three-necked flask, blow nitrogen into it and stir for 20min, slowly Add 7.8mL of n-butyllithium n-hexane solution (2.5M, 0.0195mol) dropwise, drop it over in 0.5 hours, control the te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com