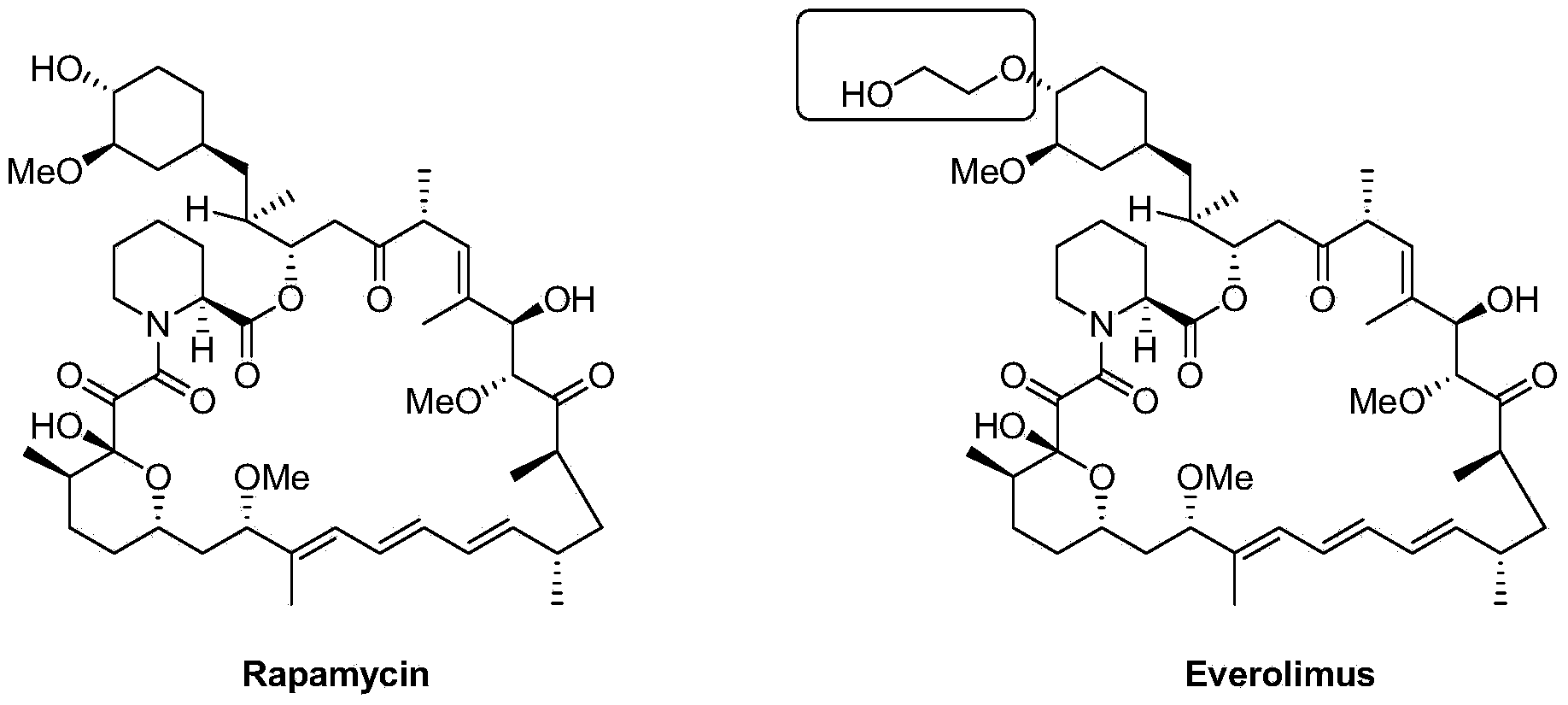
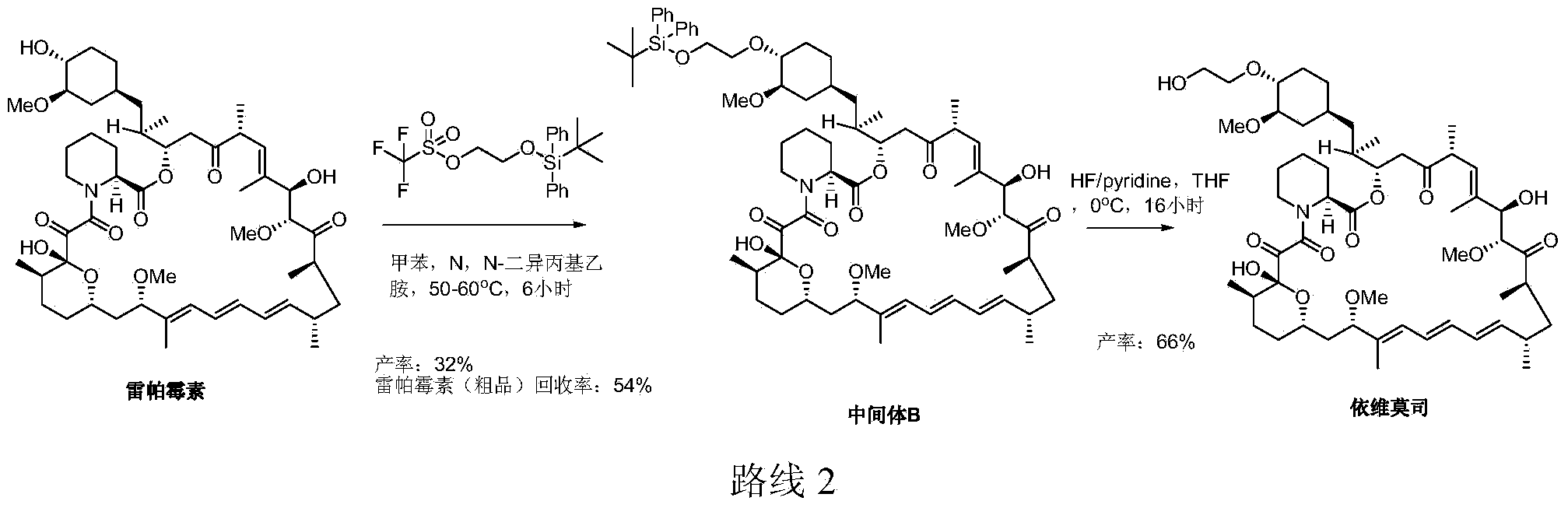
Preparation technology for everolimus
A preparation process and organic base technology, applied in the field of everolimus preparation process, can solve the problems of low conversion rate and rapamycin utilization rate, unfavorable everolimus, unrecoverable and other problems, and achieve stable and reliable reaction results , reduce degradation, improve the effect of reaction conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
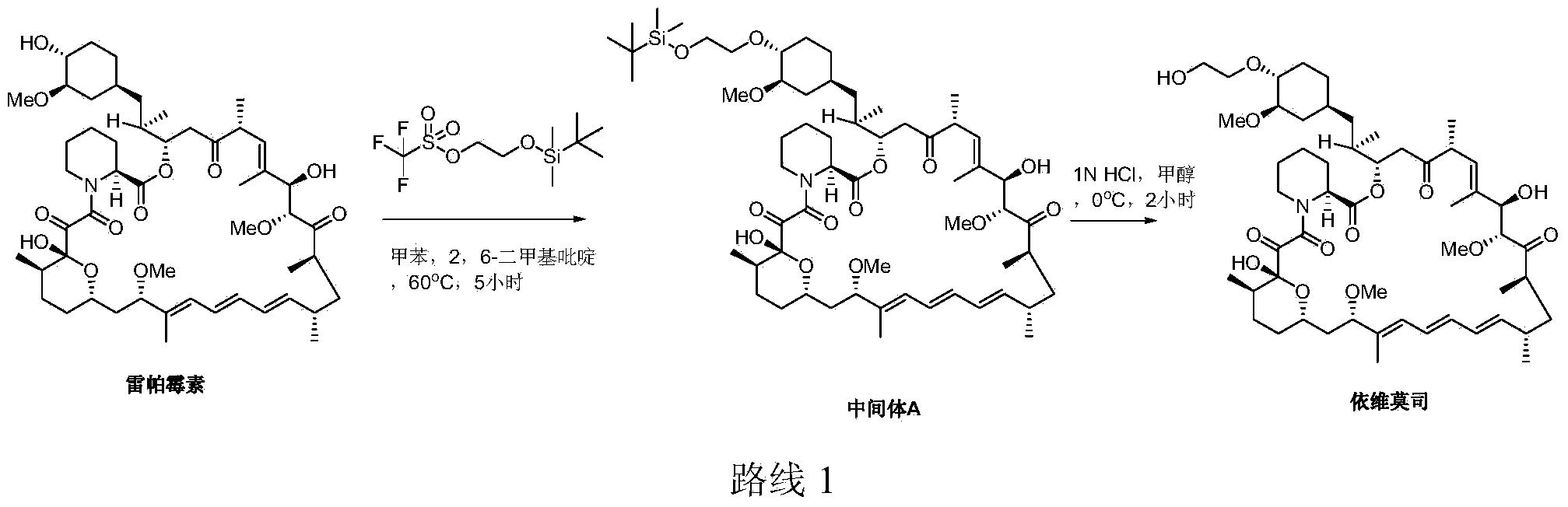
[0029] Embodiment 1. Synthesis of Intermediate A
[0030] Dissolve rapamycin (29.99g) and N,N‐diisopropylethylamine (25.75g) in toluene (80mL), heat to 60°C, add trifluoromethanesulfonic acid 2‐(tert-butyl Dimethylsilyloxy)ethyl ester (48.44g), reacted for 4.5h, and concentrated under reduced pressure to obtain a crude product. The crude product was purified by column chromatography to obtain intermediate A (23.35 g, yield: 66.4%), and rapamycin (5.03 g, recovery: 16.8%) was recovered. Example 2. Synthesis of Everolimus
Embodiment 2
[0031] Dissolve intermediate A (12.98g) in 240ml of acetone, add 48ml of 0.5M hydrochloric acid, react at 20°C for 0.5h, extract with ethyl acetate, and concentrate to obtain crude everolimus (12.35g), HPLC Chromatographic detection showed that the purity of the crude product was greater than 90%. 1HNMR (CDCl3): δ0.71(1H, dd), 0.83~1.60(m), 1.64(3H, s), 1.73(3H, s), 1.75~2.40(m), 2.52‐2.90(m), 3.00 ~3.20 (5H, s and m), 3.32 (3H, s), 3.35‐3.48 (5H, s and d), 3.52‐3.95 (8H, m), 4.18 (1H, m), 5.08‐5.60 (4H, m), 5.84‐6.40 (4H, m).
Embodiment 3
[0032] Example 3. Synthesis of Everolimus
[0033] Dissolve intermediate A (1.00g) in 20ml of acetone, add 4ml of 0.5M hydrochloric acid, react at 35°C for 0.25h, extract with ethyl acetate, concentrate to obtain crude everolimus (0.98g), and perform high performance liquid chromatography Tests showed that the crude product had a purity of 82%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com