Preparation method of ezetimibe intermediate
An ezetimibe and intermediate technology, applied in the field of pharmaceutical synthesis, can solve the problems of easy detachment of silane protective group, difficult product purification, low product purity, etc., and achieves the effects of low cost, easy nature and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
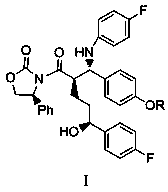
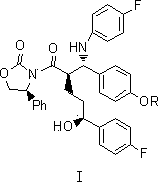
[0037] 3-[(2R,5S)-5-(4-fluorophenyl)-2-[(S)-(4-fluoroanilino)(4-Roxyphenyl)methyl]-5-hydroxypentyl Acyl]-(4S)-phenyl-2-oxazolidinone (Compound I, R=C 6 h 5 CH 2 )
[0038]
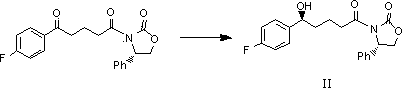
[0039] At room temperature, sequentially add (4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1,3-oxazone into a four-neck flask Heterocyclopentane-2-one (II) 30g and 4-benzyloxybenzylidene-4-fluoroaniline (III, R=C 6 h 5 CH 2 ) 30g, 25ml diisopropylacetamide and 500ml dichloromethane. After the system is mixed evenly, add 15ml of trimethylchlorosilane dropwise to the system at an internal temperature of 0°C, and add 15ml of a solution of titanium tetrachloride and isopropyl titanate to the system dropwise at -20°C (mol Ratio: 3:1), and stirred at -15°C for 20 hours after the dropwise addition. After the reaction was completed, 1000 ml of 1N hydrochloric acid aqueous solution was slowly added dropwise to the system, followed by 500 ml of dichloromethane, and the organic phase was obt...
Embodiment 2
[0042] 3-[(2R,5S)-5-(4-fluorophenyl)-2-[(S)-(4-fluoroanilino)(4-Roxyphenyl)methyl]-5-hydroxypentyl Acyl]-(4S)-phenyl-2-oxazolidinone (Compound I, R = C 6 h 5 CH 2 OCO)
[0043] At room temperature, sequentially add (4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1,3-oxazone into a four-neck flask Heterocyclopentane-2-one (II) 30g and 4-benzyloxybenzylidene-4-fluoroaniline (III, R=C 6 h 5 CH 2 OCO) 30g, 25ml diisopropylacetamide and 500ml chloroform. After the system is evenly mixed, add 15ml of trimethylchlorosilane dropwise to the system at an internal temperature of -10°C, and add 15ml of a solution of titanium tetrachloride and isopropyl titanate to the system dropwise at -20°C ( molar ratio: 1:1), and stirred at -15°C for 20 hours after the dropwise addition. After the reaction was completed, 1000 ml of 1N acetic acid aqueous solution was slowly added dropwise to the system, followed by 500 ml of dichloromethane, and extracted to obtain an organic phas...
Embodiment 3
[0046] 3-[(2R,5S)-5-(4-fluorophenyl)-2-[(S)-(4-fluoroanilino)(4-Roxyphenyl)methyl]-5-hydroxypentyl Acyl]-(4S)-phenyl-2-oxazolidinone (Compound I, R=p-BrC 6 h 5 CH 2 )
[0047] At room temperature, 30 g of compound (II) and 4-p-bromobenzyloxybenzylidene-4-fluoroaniline (III, R=p-BrC 6 h 5 CH 2 ) 60g, 25ml triethylamine and 500ml toluene. When the system is evenly mixed, add 15ml of trimethylchlorosilane dropwise to the system at an internal temperature of 0°C, add dropwise 10ml of titanium tetrachloride solution at -30°C, and stir at -30°C for 10 hours after the addition is complete . After the reaction was completed, 1000 ml of 1N hydrochloric acid aqueous solution was slowly added dropwise to the system, followed by 500 ml of toluene, and the organic phase was obtained by extraction. After the organic phase was dried over anhydrous magnesium sulfate, the organic solvent was removed to obtain a yellow solid, which was crystallized in ethanol to obtain 44 g of a wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com